All News
Australia Allows Pharmacy Substitution with Biosimilars Without Notice
Biosimilars are copies of the biologic medicines used to treat inflammatory and immunologic conditions such as rheumatoid arthritis, multiple sclerosis and cancer- potentially at lower costs.
Read ArticleCommon HIPAA Mistakes in Practice
This review details nine of the most common compliance missteps physicians are making regarding protected health information (PHI). These errors may result in legal trouble, but are avoidable. These include:
Read ArticleFDA News; Undeclared Dexamethasone Containing OTC Products
The FDA has issued public warnings that 4 over-the-counter products promoted for pain relief contain potentially harmful, undeclared dexamethasone. The supplements are Asihuri Plus Forte, Ginseng She Lian Wan, Jianbu Huqian Wan, and Saurean Fong Sep Lin.
Read ArticleMedicare D and Costly Biologics
25% of Medicare beneficiaries with rheumatoid arthritis use high-cost biologics. Yazdany and coworkers have analyzed a national cohort of beneficiaries and compared Medicare part D plans.
Read ArticleAmgen Halts Development of Brodalumab
IL-17 inhibitors are being developed for treatment of psoriasis, psoriatic arthritis and axial spondyloarthritis. Broadalumab has been in phase II & III trials and been developed by a partnership between Amgen and Astra-Zeneca.
Read ArticleAmgen & Abbvie Increase the Price of TNF Inhibitors
The manufacturers of the 2 best selling TNF inhibitors, Enbrel and Humira, have announced a 9.9% price increase of May 1st. The current average price of Enbrel is $42,000 per annum.
Read Article
A Microneedle Pill as an Alternative to Injectable Drug
Carl Schoellhammerhas just won the Lemelson-MIT National Collegiate Student Prize for his invention - the microneedle pill.
Read ArticleTanezumab in Osteoarthritis: Data Presented at OARSI
Pooled analyses of the tanezumab (a nerve growth factor inhibitor) phase 3 studies was presented at the 2015 Osteoarthritis Research Society International (OARSI) World Congress.
Read ArticleHouse Bill Threatens CME Exemption from Sunshine Act Reporting
Current Sunshine Act disclosures do not apply to CME certified educational activities for either the participants or the presenters. CMS has debated and waffled on whether companies would be required to report payments to doctors who speak at, or attend, CME seminars.
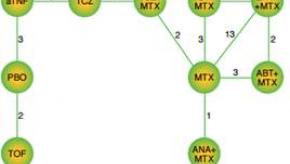
Read ArticleIs There a Standout When Comparing Novel Therapies?
Randomized clinical trials are needed to show efficacy and safety, and are needed to back up the claims and indications for a particular drug. The issue of treatment choice has become challenging, especially when choosing to start or augment therapy.
Read ArticleMedical Marijuana for Osteoarthritis in Illinois
An advisory board has recommended osteoarthritis and a host of disorders for inclusion in the Illinois new medical marijuana program.
Read ArticleMost Prescribed Branded Drugs Through March 2015
In the last 12 months, levothyroxine (Synthroid) was the most prescribed drug in the USA with 21.6 million prescriptions. Adalimumab (Humira) had 8.3 billion in sales, followed by etanercept (Enbrel) at nearly $6 billion.
Read Article
Who Should Pay for Drug 'Take-Back' Programs
Unused pills and prescriptions pose a significant safety risk in the home and may contribute to dosing errors or inadvertent drug use. The issue has led the DEA to sponsor national drug disposal programs and local municipalities have followed with similar programs. At issue is who shoul
Read Article