All News
ACR Concerns on the Scientific Integrity at the CDC
On behalf of the American College of Rheumatology (ACR), we express deep concern over recent developments at the Centers for Disease Control and Prevention (CDC) that appear to undermine the agency’s longstanding commitment to science-based public health policy.
Read ArticleILD Begins (8.29.2025)
Dr. Jack Cush reviews the news and journal reports from RheumNow.com. This week news on vaccines, safety of acetaminophen and more.
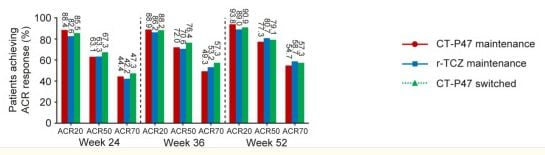
Read ArticleLong-term Lab Monitoring in RA is Inefficient
Annals of Internal Medicine has published a retrospective study of long-term routine laboratory toxicity monitoring (lt-RLTM) in patients receiving disease-modifying antirheumatic drug (DMARD) therapy showing that most very abnormal laboratory finding occur early in therapy (first 6 months) or are anticipated or only after dose escalation.
Read ArticleMethotrexate intolerance in rheumatoid arthritis
Methotrexate (MTX) is the most commonly used DMARD in rheumatoid arthritis (RA); however, there is significant intolerance to this drug among adult RA patients - especially at doses above 15 mg per week.
Read ArticleSjögren's Graduates (8.22.2025)
Dr. Jack Cush reviews the news, journal reports, FDA approvals, drug safety, and more from this week.
Read ArticleIt's now called Sjögren Disease!
Sjögren syndrome is now Sjögren disease, according to recommendations stemming from the 2023 International Rome consensus for the nomenclature of Sjögren disease.
Read ArticleSafety of Combination Targeted Therapies in Psoriatic Arthritis
Treating psoriatic arthritis (PsA) patients with more than one targeted therapy appears to be just fine, with no increased risk of serious infections, insurance claims data indicate.
Read ArticleRheums Speak: Future of Rheumatoid Arthritis
The third survey in this series addressed clinician views on future trends (AI), future therapies and lessons learned.
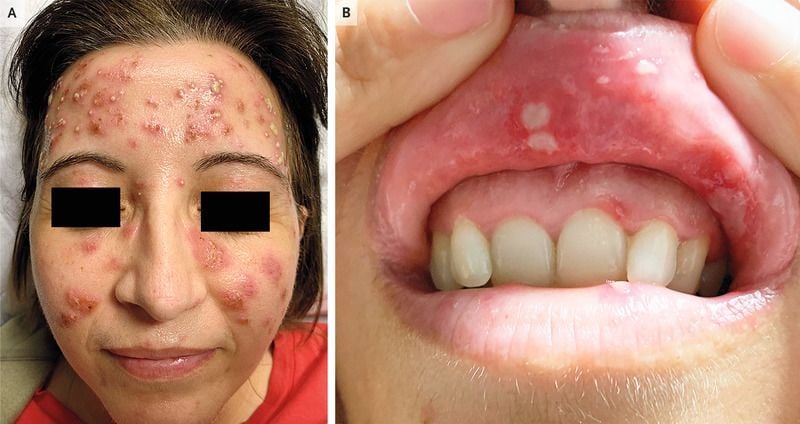
Read ArticleUncertainty with immunosuppressive for idiopathic inflammatory myopathies
A current Cochrane review suggests a continued unmet need regarding the status and utility of targeted immunosuppressant and immunomodulatory treatments for the idiopathic inflammatory myopathies (IIM). Overall there has been little progress since the previous Cochrane review (2012) that found little or no evidence to guide treatment.
Read Article

Dr. John Cush RheumNow ( View Tweet)