All News
Prevalence of Autoimmune Diseases in the United States
An epidemiologic assessment of the prevalence of autoimmune diseases in the United States (US) finds that nearly 5% of the population has an autoimmune disorder, twice as many in women compared to men.
Read ArticleAnti-Zoster Drug Prevents Shingles in Lupus Patients on Anifrolumab
The antiviral drug valaciclovir (Valtrex), given prophylactically, kept patients with systemic lupus erythematosus (SLE) under treatment with anifrolumab (Saphnelo) from having herpes zoster attacks, researchers said, offering a potential alternative to zoster vaccination.
Read ArticlePhase 3 Trial Results of Deucravacitinib in Psoriatic Arthritis
Bristol Myers Squibb announced recently the results from POETYK PsA-1 and POETYK PsA-2, Phase 3 trials pivotal trials of deucravacitinib (DEUC) in adults with active psoriatic arthritis. Both trials met their primary endpoint, demonstrating ACR20 efficacy after 16 weeks of treatment with DEUC compared with placebo.
Read ArticleBenefit of Achieving Target Serum Urate Levels in Gout with Chronic Kidney Disease
A cohort study of gout patients with CKD stage 3 found that lowering serum urate (SUA) level to less than 6 mg/dL, lowered the risk for severe or end-stage kidney disease progression.
Read Article


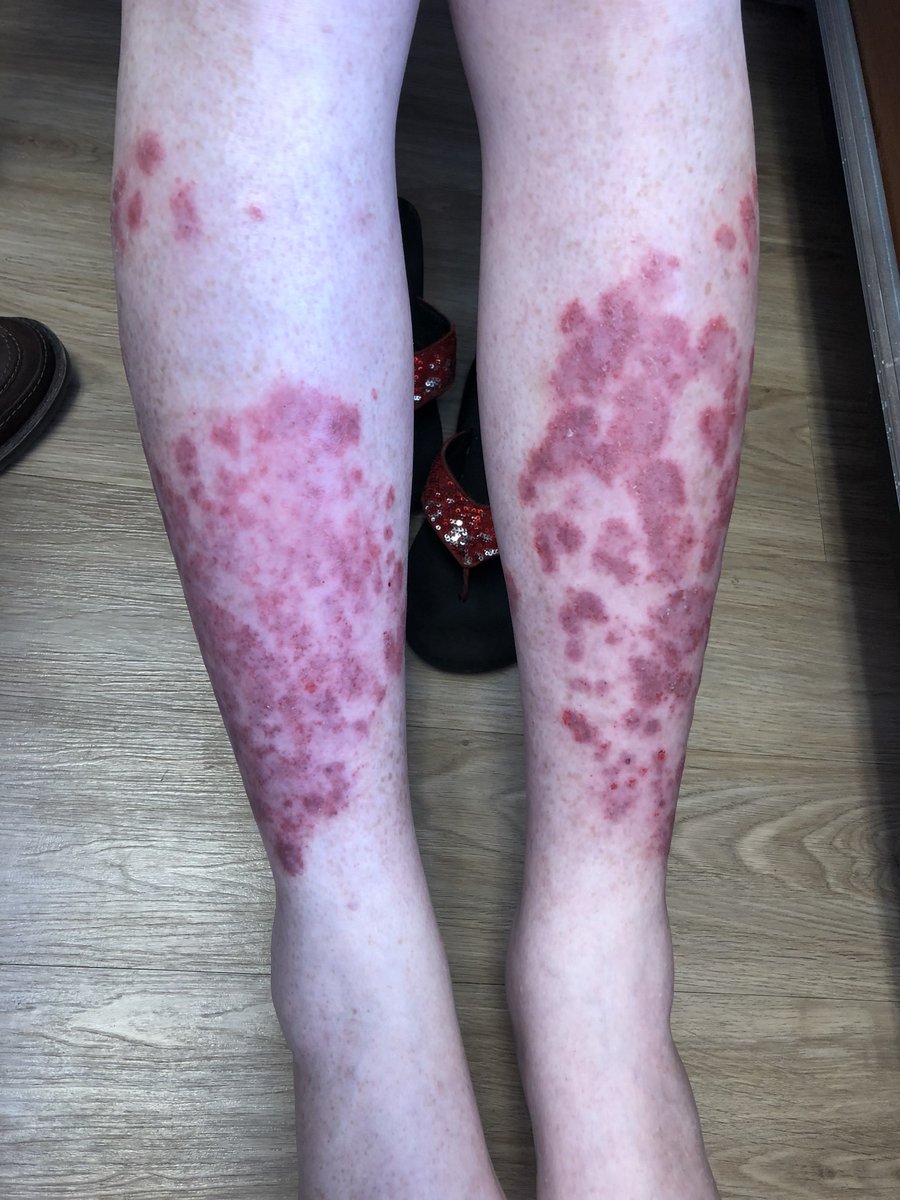
Dr. John Cush RheumNow ( View Tweet)



Links:


Dr. John Cush RheumNow ( View Tweet)


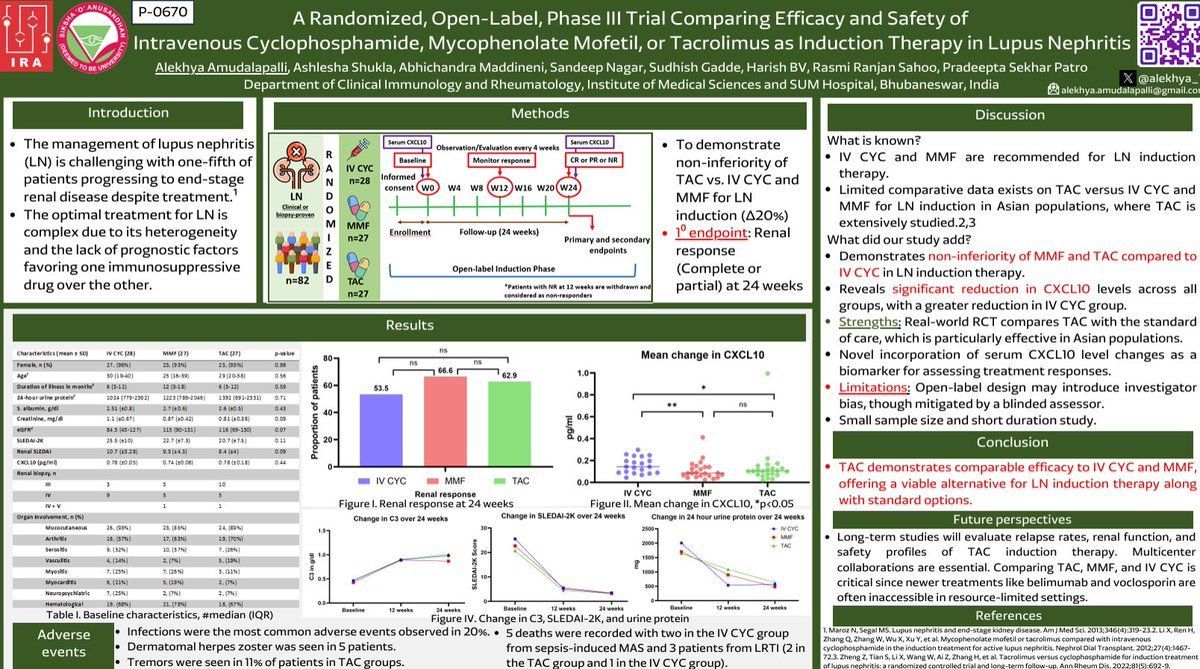
Links: