All News
Bad Actor Cofactor (10.17.2025)
Dr. Jack Cush reviews the news and journal reports, discusses pediatric reports, pregnancy data, diet and nutrition and more.
Read ArticleTelitacicept Effective in Systemic Lupus Erythematosus
The NEJM has published a report from van Vollenhoven et al showing a new dual B cell inhibitor, telitacicept, to be effective when given to active systemic lupus erythematosus (SLE) patients; but this comes with a few safety concerns.

From the plenary to posters, RheumNow is your companion for ACR Convergence 2025. Expect:
• KOL videos
• Clinical trial highlights
• Daily faculty recaps
• Live streaming
#ACR25 #Rheumatology #ClinicalTrials https://t.co/zZAwYINmC2
Dr. John Cush RheumNow ( View Tweet)

“Never in the field of human conflict was so much owed by so many to so few.” –
Winston Churchill https://t.co/ZAcYBpOmjy
Dr. John Cush RheumNow ( View Tweet)
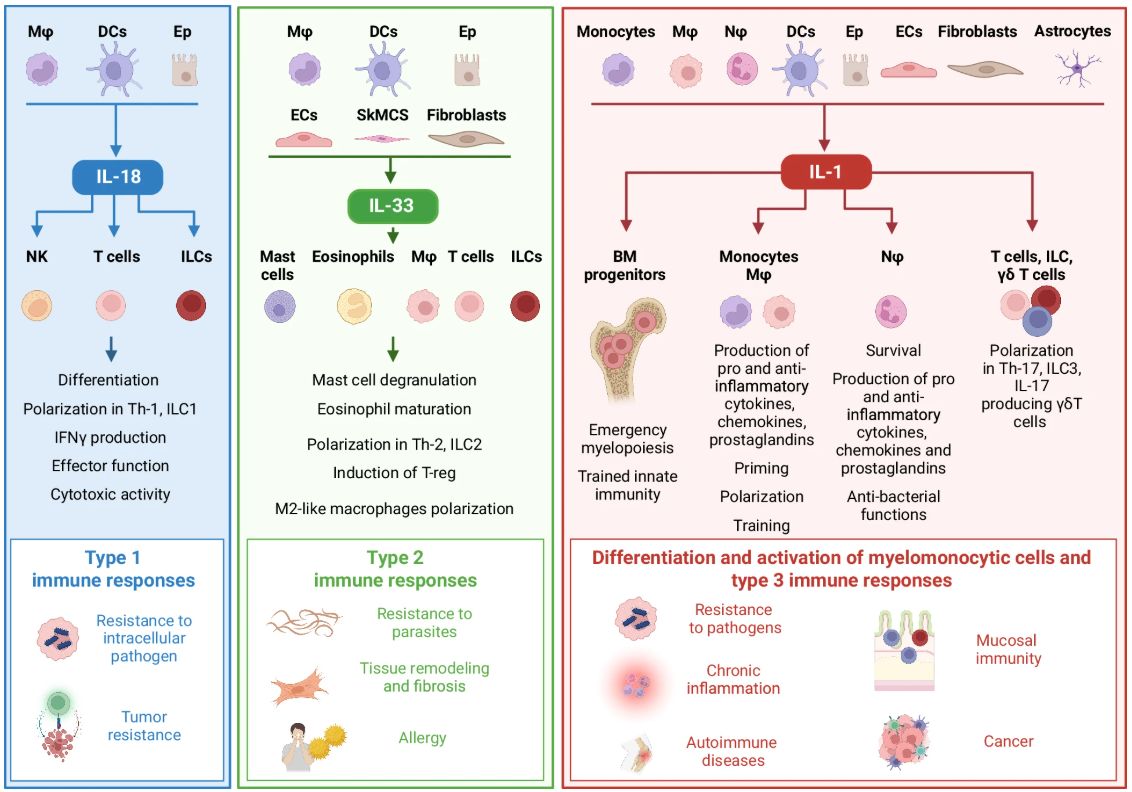
Review of IL-1 family cytokines in inflammation and immunity - including, IL-1, IL-18, IL-33, IL-36, IL-37, IL-38, IL-1Ra, etc. Full read -- Good Reference!! https://t.co/m2CwDydnB0 https://t.co/h90bdzv3y7
Dr. John Cush RheumNow ( View Tweet)

Mediterranean Diet Efficacy in Psoriasis
A small clinical trial found that a 16-week Mediterranean diet reduced psoriasis severity, presumably due to anti-inflammatory benefits of extra virgin olive oil, plant-based foods, and moderate meat consumption, according to a study https://t.co/fiJTx6TEZq
Dr. John Cush RheumNow ( View Tweet)
Whole Body MRI for Arthritis
Whole-body MRI (wbMRI) reviews have increasingly appeared in the medical literature, owing to better scanners, faster imaging times and reduced costs. Use of such non-oncologic musculoskeletal imaging provides high-contrast resolution images of the https://t.co/RQBPDhf1Vb
Dr. John Cush RheumNow ( View Tweet)

Fibromyalgia in PsO & PsA is linked to greater treatment complexity and shorter biologic therapy survival. Retro matched 1:4 cohort study 61K PsO& 244K controls. FM prevalence 3.3% (OR 1.45). In PsA decr biologic survival (6 vs 10yrs) w/ incr switching (HR 1.82)
Dr. John Cush RheumNow ( View Tweet)
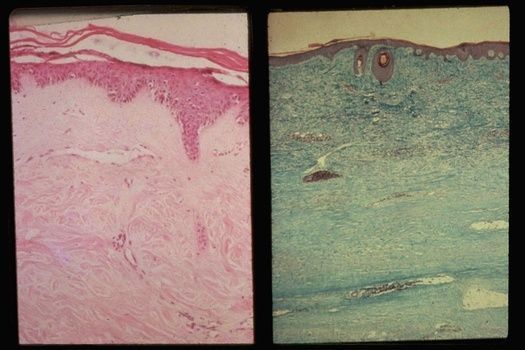
Skin Biopsies to Predict Scleroderma Outcomes?
Researchers from the Hospital for Special Surgery and Weill Cornell Medicine have shown that skin biopsies in patients with systemic sclerosis (SSc) can define a fibroblast immunophenotype that may have prognostic and early https://t.co/z3LykOgyHO
Dr. John Cush RheumNow ( View Tweet)

CONGRATULATIONS: Dr. Lauren Henderson has been awarded the Gale & Ira Drukier Prize in Children’s Health Research. Lauren is a pediatric rheum at Boston Children’s Hospital, focused in difficult JIA. She has studied T-B cells interactions & long-term joint damage in JIA https://t.co/uYWrh0oasy
Dr. John Cush RheumNow ( View Tweet)

Metanalysis of whole-body cryotherapy (WBC) in Ankylosing spondylitis (AS) - 5 studies & 310 #AS pts. Temps (−60°C to −10°C) & duration (80 sec to 3 min) varied. WBC significantly mproved BASDAI (p<0.001), ASDAS ( 0.015), BASFI (0.006), & VAS pain (.005), but NOT CRP https://t.co/6Sj3bg096X
Dr. John Cush RheumNow ( View Tweet)

A high ANA titer (≥1:640) in individuals without an autoimmune disease was found to be strongly associated with liver-related disorders, including nonalcoholic and alcoholic liver disease
Arthritis Care & Research
https://t.co/tkMpPFPnhc https://t.co/Bk9JXWTsOU
Links:
ACR_Journals ACR_Journals ( View Tweet)

JAMA Pediatrics study of 1,473 young US adult, finds 1/4 use cannabis or alcohol for sleep (18% cannabis; 7% EtOH), even though these may interfere with quality or ability to stay asleep. In those using cannabis in the last yr, 41 reported using cannabis to sleep. https://t.co/fdW6bdDM3I
Dr. John Cush RheumNow ( View Tweet)

Arthritis Pain and Education Gaps Linked Save
New research from The University of Texas at Arlington shows that differences in state welfare policies are linked to rising arthritis-related joint pain across much of the U.S.
https://t.co/cGRH9X6pMZ https://t.co/J1SDeKrytn
Dr. John Cush RheumNow ( View Tweet)
Failure of IL-17 inhibition in Lupus nephritis. 31 LN pts enrolled in phase 2 DBRPCT of secukinumab 300 mg IV, but was terminated early for futility (complete renal response at week 52 was lower with secukinumab (24.2%) than with placebo (36.3%). https://t.co/471TolqtFk https://t.co/gKi2Z2MZHS
Links:
Dr. John Cush RheumNow ( View Tweet)
Muscle mass & JAKi? 15 active RA pts (+ sarcopenia risk) Rx w/ tofacitinib - signif. improved @1 mo. @6 mos muscle mass signif improved (+242 cm3, +4%, p=0·017) {less w/ NSAIDs); w/ signif creatinine incr (0.06 mg/dl) & decr in serum IL-6, IL-1 & TNF. No change in CK, myoglobin, https://t.co/taY7DisMOS
Dr. John Cush RheumNow ( View Tweet)
Why Brittle Bones Aren't Just a Woman's Problem
Osteoporosis is more common in women, prompting routine screenings for them at age 65. As a result, men might overlook getting a scan, which may not be suggested by an orthopedic specialist. However, about 20% of men over 50 will https://t.co/ggyse5ZDPV
Dr. John Cush RheumNow ( View Tweet)

Pregnancy Management in Inflammatory Bowel Disease: A Global Consensus
Consensus guidance on the management of pregnancy in patients with inflammatory bowel disease (IBD) was simultaneously published in six international journals, including The American Journal of https://t.co/F6er2mpUoV
Dr. John Cush RheumNow ( View Tweet)

BMJ Systematic review of 217 RCTs w/ 15 684 pts shows most beneficial exercises for Knee Osteoarthritis are aerobic activities such as walking, cycling, or swimming significantly mitigated pain during both short and mid-term follow-up periods https://t.co/WmQBFNis6t https://t.co/KXv2mogvZx
Dr. John Cush RheumNow ( View Tweet)

Osteonecrosis: suspect with trauma, high-dose steroids, alcoholism, renal failure, transplantation, SLE, thrombotic states (e.g., sickle cell anemia, hemoglobinopathies), radiation injury, pancreatitis, gout, pregnancy, hyperlipidemia, & caisson disease (decompression sickness) https://t.co/y6iYd5fWZ2
Dr. John Cush RheumNow ( View Tweet)





