All News
Increasing Corticosteroid Use in Non-Hospitalized COVID-19
JAMA reports that despite NIH recommendations (that corticosteroids only be used in hospitalized COVID-19 patients), nonhospitalized COVID infected patients were oftent prescribed systemic corticosteroids.
Read ArticleTo TDM or Not in Rheumatology?
Therapeutic Drug Monitoring (TDM), especially for biologic therapies, has become a standard in gastroenterology, but shunned by most rheumatologists.
Read ArticleCan SGLT2 Inhibitors Be Protective in Lupus and Vasculitis?
SGLT2 inhibitors are all the buzz in the renal world with reports of a renal protective and mortality lowering effects. Maybe these agents should be considered and studied in lupus nephritis and ANCA- associated vasculitis.
Read ArticleAmitriptyline and FDA Treatments for Fibromyalgia
Both old and newer pharmacological treatments for fibromyalgia are often promoted; but a recent systematic review and network meta-analysis showed that duloxetine had higher efficacy in pain and depression, while amitriptyline had higher efficacy in treating sleep, fatigue, and quality of life outcomes.
Read ArticleBiologic Persistence in Psoriatic Arthritis and Psoriasis
Two recent studies examined the durability and persistence of biologic agents in patients with psoriasis (PSO) and psoriatic arthritis (PsA) and while some differences were noted overall persistence was low 3 years later.
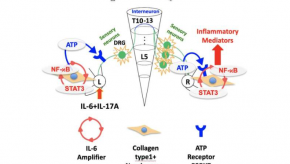
Read ArticleInflammatory Arthritis Spread by ATP and Neural Crosstalk
A novel study from the Journal of Experimental Medicine suggests that a reflex neural signaling at sites of inflammation may lead to spread to other inflammatory sites via and ATP-mediated neural crosstalk.
Read ArticleRNL 2022: Spondyloarthritis Spectrum
This RheumNow Live 2022 session features highlights from lectures on Spondyloarthritis.
- Microbiome in Spondyloathritis - Jose Scher, MD
- Axial Psoriatic Arthritis - Philip Mease, MD
- Juvenile Spondyloathritis - Pamela Weiss, MD
Read Article
Multimorbidity Burden in Rheumatoid Arthritis
Crowson and colleagues at the Mayo Clinic have shown that rheumatoid arthritis (RA) patients have a higher prevalence of multimorbidity with as many as 44 different morbidities of interest in RA.
Read ArticleAcute Inflammatory Responses Needed to Resolve Chronic Pain
Science Translational Medicine has published results of a Canadian study of low back pain (LBP) suggests that pain control by anti-inflammatory treatments might have negative effects on pain duration and may be counterproductive for long-term pain outcomes.
Read ArticleBimekizumab Development Delayed by FDA
UCB announced they have received a Complete Response Letter (CRL) from the U.S. Food and Drug Administration (FDA) regarding the Biologics License Application (BLA) for bimekizumab for the treatment of adults with moderate to severe plaque psoriasis (PSO).
Read ArticleMethotrexate Monitoring (5.13.2022)
Dr. Jack Cush reviews the news and journal reports from the past week on RheumNow.com. Bad news for digitial ulcers in Systemic sclerosis, Readmissions in Lupus and Thrombocytopenia in APL patients.
Read ArticleWiki Guidelines Approach to Pyogenic Osteomyelitis
A crowd sourced, WikiGuidelines approach to clinical treatment guideline development for pyogenic osteomyelitis yielded 7 important clinical questions, 2 clear treatment recommendations and 5 topic reviews to inform future treatment or investigations.
Read ArticleBimekizumab's Favorable Safety Profile in Psoriasis
A 2-year safety study of bimekizumab, the dual IL-17A/F inhibitor, plaque psoriasis patients showed bimekizumab (BMK), to be effective, well tolerated, with no new safety concerns other than a modest risk of oral candidiasis.
Read ArticleRNL 2022: Psoriatic Arthritis Advances
Tuesday Nite Rheumatology on 10 May 2022 featured session highlights from RheumNow Live 2022.
This webinar presented exerpts from these lectures along with audience Q & A
Read ArticleASCORE: Seropositivity Favors Abatacept Drug Survival
The ASCORE (Abatacept SubCutaneOus in Routine clinical practicE) study evaluated efficacy, safety, and drug retention (durability) of abatacept (ABA) moderate-to-severe rheumatoid arthritis (RA) and found consistently better ABA durability in those who were seropositive.
Read ArticleMaking You the Expert of You! (Smart Rules for Patients)
You’ve scheduled an appointment with your medical provider - how do you make the most of it? We've gathered these tips and suggestions to share with patients.
Read ArticleDiagnostic Delays in Spondyloarthritis Still Prolonged
Previously quoted average delay in diagnosing axial spondyloarthritis (axSpA) may be improving as a recent UK study shows the mean average time to diagnosis in two different NHS trusts was between five and six years.
Read ArticleWhat is JAKne? (5.6.2022)
Dr. Jack Cush reviews the news, journal reports, FDA actions, new side effects and changing prevalence of Gout.
Read ArticleLack of Research Stymies Uptake of Medical Marijuana for Rheumatic Pain
A new review article from CreakyJoints finds that there has been limited progress in understanding the potential of cannabis based therapies for the treatment of pain associated with rheumatic conditions in the past five years because of a lack of standardization of clinical research and barriers to conducting research due to existing federal and state regulations.
Read ArticleAcne with JAK Inhibitors
JAK inhibitors have become enormously popular and while their side effect profile has been delineated and reviewed, little mention is made of acne – an adverse event that may affect up to one-quarter of patients taking JAK inhibitors (JAKi).
Read Article