All News
Sequencing DNA to find new lupus treatments
Medical University of South Carolina geneticist Betty Tsao, Ph.D., will lead a five-year project to identify rare mutations associated with childhood-onset systemic lupus erythematosus (SLE), or lupus, with more than $3.5 million in funding from the National Institute of Arthritis and Musculoskeletal and Skin Diseases (NIAMS). Tsao holds the Richard M. Silver Endowed Chair for Inflammation Research in the Division of Rheumatology and Immunology at MUSC.
Read ArticleAssociations in Rheumatology (10.3.2025)
Dr. Jack Cush reviews the news, journal reports and important associations in rheumatology from the past week on RheumNow.com.
Read ArticleActivity Trackers Can Predict RA Flares
People with rheumatoid arthritis (RA) who experienced disease flares often showed changes in heart rate and physical activity captured by popular wearable devices 4 weeks before they became clinically apparent, a small study indicated.
Read ArticleILD in Juvenile Dermatomyositis: Moving Toward Better Outcomes
Juvenile dermatomyositis (JDM) is typically recognized for its striking skin rashes, vasculopathy, and muscle inflammation. Less visible, but equally significant, is lung involvement.
Read Article

Links:
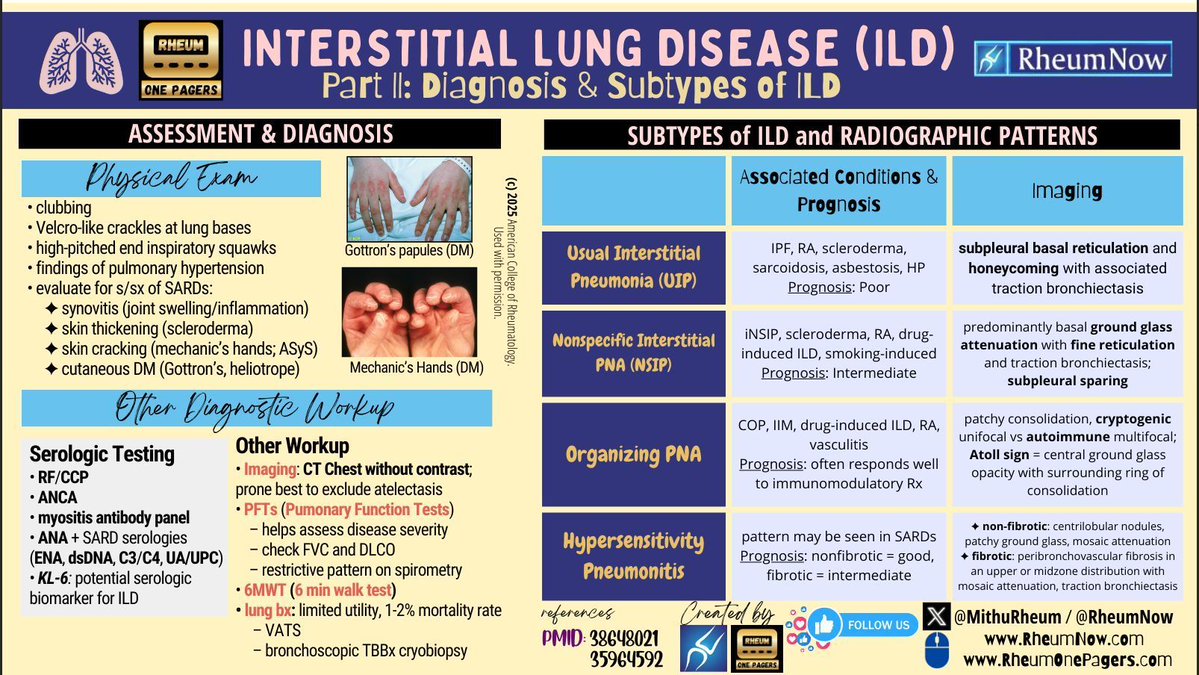
🆕 ILD (Part III): clinical practice focus 🔬 Diagnostic challenges 🧑⚕️ Case-based learning 📈 Evidence & outcomes 🌍 Global perspectives ⬇️ Download & learn more: https://t.co/WhpVzAfPDD Created by @MithuRheum | For our Rheum to Breathe: ILD Campaign https://t.co/4CL9mX9D6Y







Links: