All News
Emapalumab Approved for MAS in Still’s
On June 27th, 2025, the FDA approved emapalumab for macrophage activation syndrome (MAS) in known or suspected Still’s disease (including both adult-onset Still’s disease (AOSD) and systemic juvenile idiopathic arthritis (sJIA)) with inadequate response or intolerance to glucocorticoids.
Read ArticleRA Lung Disease Portends Poor Outcomes
A retrospective Harvard affiliates study shows that rheumatoid arthritis (RA) patients with lung disease have higher mortality, especially respiratory and infection-related mortality than those without lung disease (LD).
Pesticides and Rheumatoid Arthritis?
d
MedPage Today
Women exposed to pesticides through farm work or as farmers' wives face increased risk for developing rheumatoid arthritis, a new analysis of Agricultural Health Study data indicated.
Read Article
Retrospective review of 457 Autoimmune liver disease pts found 42.5% with Polyautoimmunity. Predictors included ANA positivity (78 vs 47%), ANA titers, dsDNA (12 vs 2%), RF+ (25 vs 9%), disease duration, low albumin, high ESRs. https://t.co/Socl6XWU9a https://t.co/8qomVBh3KP
Dr. John Cush RheumNow ( View Tweet)
Osteoarthritis (OA) degenerative or inflammatory? 296 pts w/ Knee OA (age 63 yrs, 2/3 had poor oral hygien, K&L grade 2, pain >40). Periodontitis in 62.5% & signif assoc w/ Xray K&L grade 4 (OR 5.39), poor oral hygiene (OR 34), dental visits (OR 8.54), brushing time (OR 21.9) https://t.co/xXIr2qxtt4
Dr. John Cush RheumNow ( View Tweet)

La Paz study of 903 RA pts; 136 (14.5%) were D2T-RA, 96 for inefficacy. They define early vs Late D2T-RA=44.5 mos, & differed>> Early D2T-RA (1/3) pts more likely to have anxiety or depression (76% v 0), higher CRP @6mos, & time to develop D2TRA (35 vs 70 mos) https://t.co/KFG68y9Uq5
Dr. John Cush RheumNow ( View Tweet)
Zasocitinib (Tyk2 Inhibitor) Efficacy in Psoriatic Arthritis
A highly selective, tyrosine kinase 2 inhibitor zasocitinib (TAK-279), was studied in a phase 2b trial and found to be effective and safe patients with active psoriatic arthritis (PsA).
https://t.co/HB0eVLLAh6 https://t.co/5Kqopaw6wL
Dr. John Cush RheumNow ( View Tweet)

BMS announced today that the FDA has accepted for review the supplemental New Drug Application (sNDA) for Sotyktu (deucravacitinib) for the treatment of adult active psoriatic arthritis. The FDA decision (PDUFA) date is March 6, 2026. https://t.co/yMxkUP5FAw https://t.co/2teEUbWFem
Links:
Dr. John Cush RheumNow ( View Tweet)

What's been the greatest healthcare advance in the last 20 years?
Dr. John Cush RheumNow ( View Tweet)
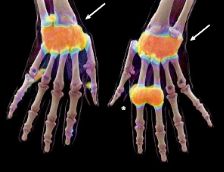
Full read overview of new imaging modalities for inflammatory arthritis, including high-res.quantitative CT, ultra-high field MRI), PET, fluorescence optical imaging, optoacoustic imaging, contrast-enhanced ultrasonography - all with artificial intelligence analysis https://t.co/rbHiPWAAFP
Dr. John Cush RheumNow ( View Tweet)

MTX lowers risk of serious infxn (SI) - UK Observational study of 17 472 newly Dx RA (2018–2023); 10K on MTX, 4,540 other csDMARDs; 13680 corticosteroids (GC). 1307 SI (3/100 PY) w/ reduced adj HR 0.72 (95% CI: 0.63–0.82). 311 SI-related mortality (IR 0.69;0.61–0.77) https://t.co/iuxAivtrYZ
Dr. John Cush RheumNow ( View Tweet)
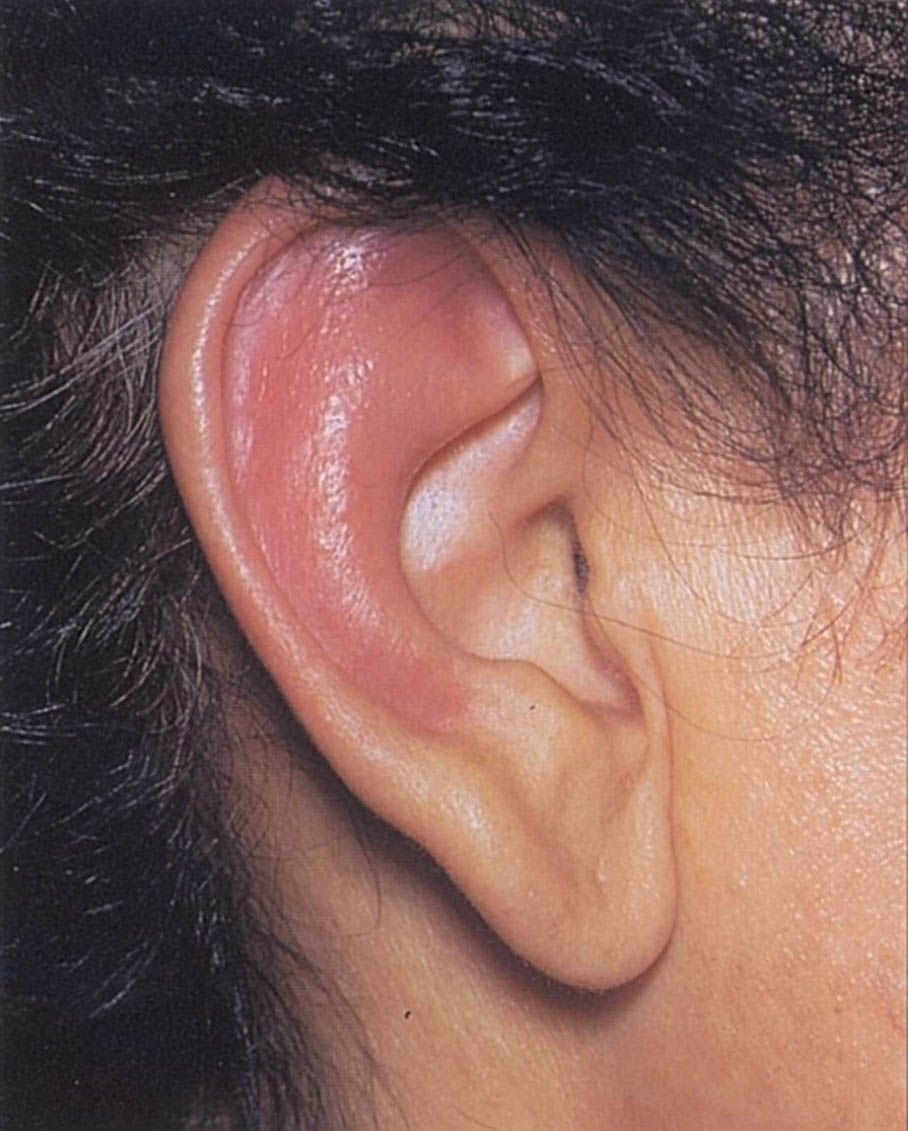
Manifestations and Treatment in Relapsing Polychondritis
A large multicenter cohort study of Relapsing Polychondritis (RP) describes the clinical manifestations and treatment approaches to this rare, heterogeneous, multisystem disease.
https://t.co/Gz2qwtC4jZ https://t.co/llK9W45DlW
Dr. John Cush RheumNow ( View Tweet)

“Never be limited by other people's limited imaginations." – Mae Jemison (Physician & first Black woman in space) https://t.co/HwMuaAUVMI
Dr. John Cush RheumNow ( View Tweet)
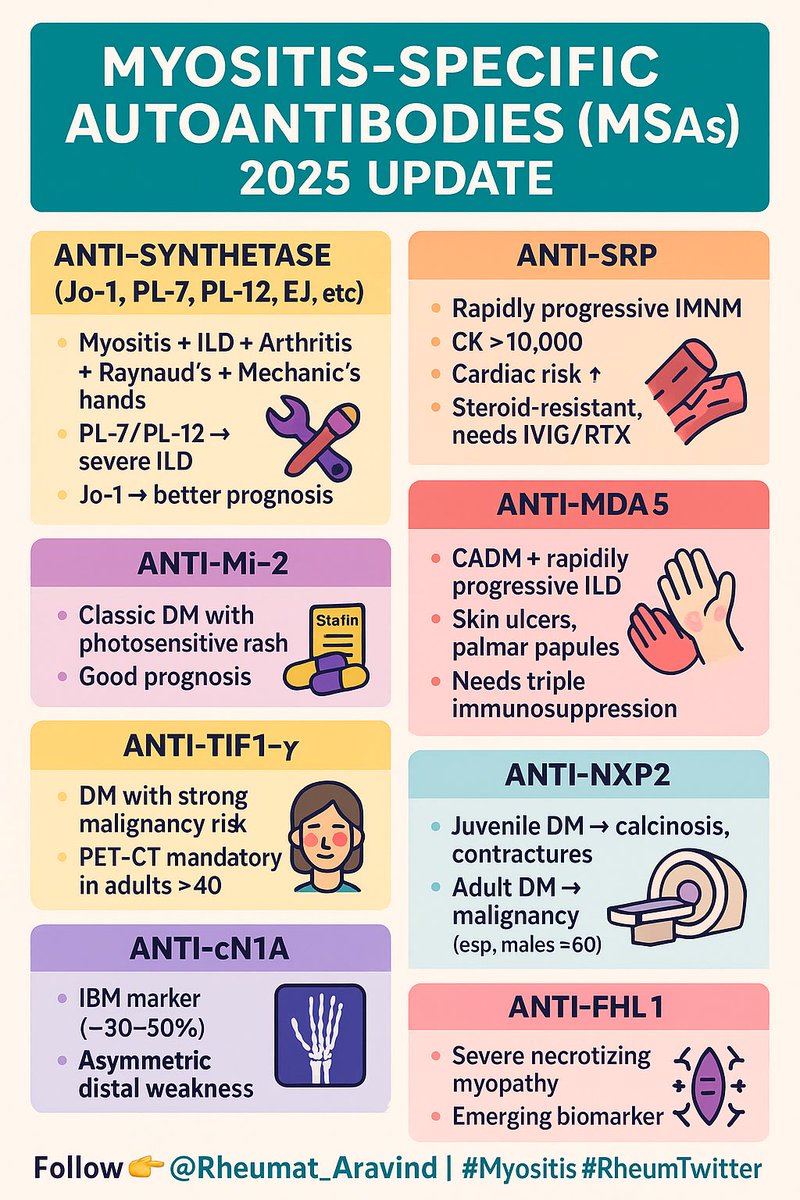
🧬 Myositis-Specific Autoantibodies (MSAs) – 2025 Update
MSAs aren’t just diagnostic—they predict phenotype, prognosis, & treatment response.
👇 Infographic #RheumTwitter #Myositis #MedEd @IhabFathiSulima @DrAkhilX https://t.co/91U5s88AdB
Aravind Palraj Rheumat_Aravind ( View Tweet)

Short full read review of the history, safety and use of Corticosteroids in Respiratory Medicine (including use in community-acquired pneumonia (CAP), ARDS, septic shock, ILD, COPD/asthma, COVID-19, Cancer, Sarcoid & IPF. https://t.co/aKMAT8IhLm https://t.co/RopoNnYJ6Z
Dr. John Cush RheumNow ( View Tweet)

SSB, > SSA, Abs are signif associated w/ abnormal DLCO (AUC 0.791) in Sjogrens syndrome, esp in assoc. w/ LIP- lymphocytic interstitial pneumonitis. These findings more likely in quadruple positive Sjogrens w/ RF/ANA/SSA/SSB positivity. https://t.co/s6IyRzfXiN https://t.co/rKDBovTOpl
Dr. John Cush RheumNow ( View Tweet)

Sulfasalazine as a preventative against Pneumocystis (PJP). 594 RA pts & 848 Rx episodes - 181 on SSZ, 667 controls. W/ 850 Pt-Yrs F/U, 21developed PCP, 3 died of PCP. SSZ significantly lowered PJP risk (p = 0.003, rate ratio = 0.05; 95% CI 0.00–0.34). https://t.co/PAgBaJGldR https://t.co/PuTxGXTeRS
Dr. John Cush RheumNow ( View Tweet)

Study of a Chinese systemic JIA cohort using scRNA-seq and Bulk RNA-seq, found a potential biomarker w. UBE2D1 expression closely related to Dz activity levels, reflecting enhanced monocyte activity in sJIA patients. UBE2D1 may complement others S100A8/A9, IL-18, ferritin https://t.co/hp7sFR6kLa
Dr. John Cush RheumNow ( View Tweet)

Biologic survival study of British Assoc. of Dermatologists Biologics Register (11/07-6/23) looked at 19,034 Rx courses (median F/U 2.3 yrs), of TNFi, IL-12/23i, IL-17i, & IL-23i. IL-23i (GUS & RIS) had best survival (~1.93yrs) for efficacy & safety (broadalumab the least) https://t.co/uhomYmiUZy
Dr. John Cush RheumNow ( View Tweet)

10-20% of IBD pts have IBD-assoc. spondyloarthritis, as either peripheral arthritis or axial Dz, subclinical gut inflammation w/ IL-23–IL-17–TNFα playing central roles. TNFi & JAKi Rx is effective, but IL-17i may exacerbate IBD (vedolizumab??) Review here>> https://t.co/EL0sL2M3YX
Dr. John Cush RheumNow ( View Tweet)