All News
Alveolar Hemorrhage and Mortality in Lupus
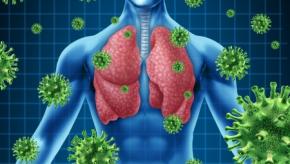
Metanalysis of patients with diffuse alveolar hemorrhage (DAH), show this to be a rare and life-threatening complication of systemic lupus erythematosus (SLE), that is more apt to affect older, severe lupus patients with active infection.
Read ArticleACR and Three Societies Agree on HCQ Eye Safety
Four major medical societies issued a joint statement of principles on safe use of hydroxychloroquine from the perspective of ocular toxicity.
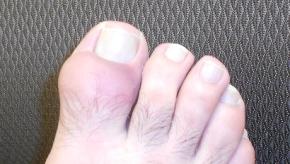
Read ArticleMIRROR Study: Pegloticase With Methotrexate in Uncontrolled Gout
Botson et al has reported that the combination of methotrexate (MTX) and IV pegloticase is safe and effective in patients with uncontrolled gout.
This exploratory, open-label clinical trial screened 17 patients and treated 14 patients (all men, 49.3 ± 8.7 years) with IV pegloticase with MTX (15 mg/week) and folic acid (1 mg/day) 4 weeks prior to and throughout pegloticase treatment.
RWCS Highlights - Day 3
Day 3 report from the 2021 RWCS meeting in Maui (and virtually) includes pearls from the PsA 2020 Year in Review; fact vs. fiction on evidence based medicine: diet and rheumatic diseases; hot topics in allergy/immunology; and pediatric rheumatology highlights.
Read ArticleWeekly Semaglutide for Weight Loss in Adults
NEJM has reported the results of a trial showing semaglutide once weekly plus lifestyle intervention was associated with sustained, weight loss in overweight or obese adults; such that nearly one-third lost over 20% of their body weight.
Read ArticleACR Promotes New COVID-19 Vaccine Clinical Guidance for Rheumatic Patients
The American College of Rheumatology has published a draft guidance on the use of COVID-19 vaccination in rheumatic disease and musculoskeletal disease patients, based on the efforts of the North American Task force. The document provides guidance to rheumatology providers on the use of the COVID-19 vaccine and the associated management of RMD patients around the time of vaccination. Here is a summary of the recommendations.
Read ArticleSynovial Tissue Signature Guiding Targeted IL-6 Therapy in RA
RNA sequencing and classification of RA subsets has proven useful. RNA sequencing-based stratification of RA synovial tissue showed stronger associations with clinical responses compared with histopathological classification. Additionally, for patients with low or absent B-cell lineage expression signature in synovial tissue, tocilizumab is more effective than rituximab.
Read ArticleComorbidity and Drugs Drive COVID-19 Severity and Survival in Rheumatic Disease
While comorbidities are associated with severe COVID-19 infection, it appears they also influence severity and survival in rheumatic disease (RMD), according to a a French cohort study,
Read ArticleRheumNow Podcast – Tofacitinib Safety Concerns (2.5.2021)
Dr. Jack Cush reviews and discusses the news and journal reports from the past week on RheumNow.com.
Read ArticleHydroxychloroquine Fails to Prevent COVID-19
There is a preponderance and mounting evidence that hydroxychloroquine is ineffective in COVID-19 infection; and now the NEJM reports a the results of a trial where HCQ given as post-COVID exposure therapy failed to prevent SARS-CoV-2 infection or symptomatic Covid-19 in healthy persons.
Read ArticleCardiac and Cancer Signals Tofacitinib Safety Alert from FDA
The FDA has notified healthcare professionals of a safety alert concerning tofacitinib (Xeljanz), noting that preliminary results from a long-term safety clinical trial show an increased risk of serious heart-related problems and cancer with tofacitinib (compared to adalimumab) when given to
Read ArticleAdverse Events with Anti-malarials during the COVID Pandemic
For a variety of reasons, the use of anti-malarials (chloroquine and hydroxychloroquine) rose dramatically in the pandemic; notable was the lack of proven benefit and the dramatic risk in reported adverse drug reactions (ADRs) associated with these drugs being used to treat SARS-CoV-2.
Read ArticleHealth Canada Approves Subcutaneous Infliximab Biosimilar
The infliximab biosimilar CT-P13, developed by Celltrion and marketed as Remsima has been approved by HealthCanad for subcutaneous (SC) use in all the indications for infliximab.
Read ArticleLow Persistence of Biologics in Psoriatic Arthritis
A longitudinal observational cohort study of psoriatic arthritis (PsA) patients treated with biologic therapy finds relatively low persistence of selected biologic therapy in PsA patients
Read ArticleVagal Nerve Stimulation in Rheumatoid Arthritis
Lancet Rheumatology has reported the results of a proof of concept trial, wherein vagal nerve stimulation was shown to ameliorate RA disease activity. The intervention involved non-invasive stimulation of the auricular branch of the vagus nerve using a wearable vagus nerve stimulation device for up to 30 min per day, delivering pulses of 20 kHz.
Read ArticleOne-Third Rheumatoid Risk from Lifestyle
Analysis of the NHANES (National Health and Nutrition Examination Survey) data suggests that one-third of the risk of developing rheumatoid arthritis (RA) in the USA is attributable smoking, obesity and alcohol intake.
Read ArticleCalculating Serious Infection Risk in IMID Patients
Predictive modeling of data derived from the DANBIO registry (of treated IMID patients) revealed a 4 fold increased risk of serious infection (SIE) in those starting biologic DMARD (bDMARD) treatment. From this large dataset, researchers developed a simple prediction model to estimate future infection risk that may inform shared decision-making in individual patients.
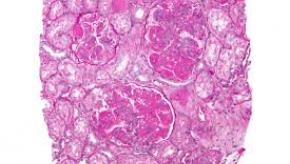
Read ArticleLupus Nephritis Despite Low Level Proteinuria
Kidney International reports on a series of systemic lupus erythematosus (SLE) patients, who despite inactive urinary sediment and low level proteinuria, had a high rate of glomerulonephritis (GN) proven by renal biopsy; moreover, the LN was not predicted by laboratory abnormalities.
Read ArticleJAK Inhibitor Misses Endpoint in Safety Study. Now What?
Pfizer announced results Wednesday from its FDA-mandated postmarketing safety study of tofacitinib (Xeljanz), and they don't bode well for the drug and possibly others in its class.
Read ArticleUrate Lowering Therapy During Acute Gout
Acute gout has its well defined protocols, and most state that urate lowering therapy (ULT) should be continued; but does ULT affect outcomes in an acute gout attack?
Read Article