All News
Rheumatology Round-Up with Drs. Kavanaugh & Cush
The Annual Review of Best ACR 2020 abstracts featuring Drs. Cush and Kavanaugh filmed on the last day of Virtual ACR 2020 Nov. 9, 2020. Watch now!
Read Article
Rheumatology Round-Up 2020 Tonight!
Rheumatology Round-Up tonight 8:00pm EST, 7pm CST. Drs. Artie Kavanaugh and Jack Cush reprise their long-running end of ACR highlights. Rheums can watch by Zoom (Q&A too), everyone can watch on RheumNow.com or our YouTube channel. See you there!
Read ArticleACR20 - Day 3 Report
Here are my highlights from day three, Sunday, of the ACR 2020 Convergence.
Read ArticleACR20 - Day 2 Report
Saturday was the day 2 with many posters and live sessions at the 2020 Virtual ACR/ARP meeting. Here are a few of my highlights from day two:
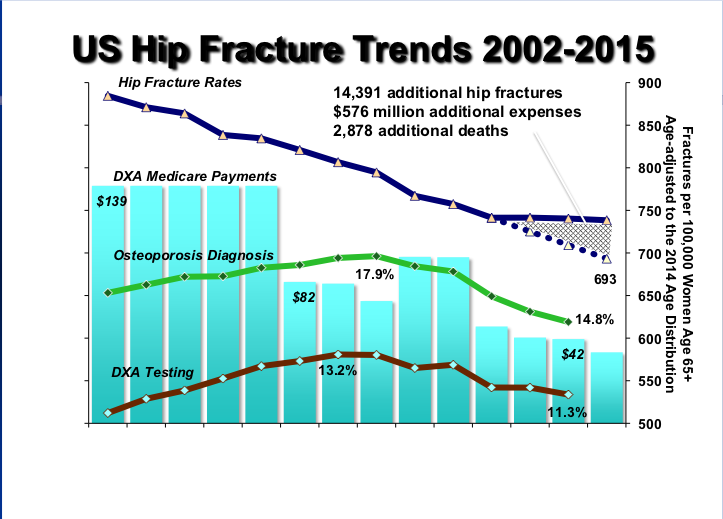
Read ArticleTNF Inhibitor Associated Infectious Risk Greatest in First 6 Months
The risk of serious infections (SIE) in RA patients given anti-TNF (TNFi) therapy is not static, instead it has been shown to be greatest in the first 6 months of use according to a recent report in Rheumatology.
Read ArticleACR 2020 Your Way - Get Your Topic Emails
ACR is but a few days away; the question is: how will you tackle this virtual meeting? RheumNow has created several options for learning the way you want to. First is to feed your need. Are you a lupologist? A gout maven? A Spondylitis sleuth? A drug safety wannabe?
Read ArticleLong-Term Benefits of Ixekizumab in Psoriatic Arthritis
The SPIRIT-P1 study has demonstrated the safety and efficacy of ixekizumab (an IL-17A antagonist) when given to adults w/ active psoriatic arthritis (PsA), and also demonstrating radiographic protection and improvement in skin outcomes as well.
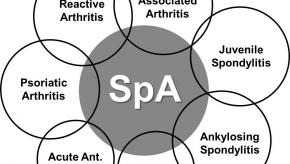
Read ArticleSPARTAN ACR20 Recommendations on Spondyloarthritis
SPARTAN has compiled a list of presentations and sessions worth seeing if you are interested in ankylosing spondylitis, spondyloarthritis and related disorders.
Read ArticleUS Differs from Worldwide Drug Reimbursements
"The US has nearly 4.5% of the world’s population but accounts for more than 40% of global drug spending" reports JAMA Internal Medicine. US drug prices are nearly double those of Australia, Canada, and the United Kingdom, and the US spends twice as much on retail and nonretai
Read ArticleAre Rheums Attending ACR 2020?
ACR 2020 (aka ACR Convergence) is just around the corner, with the opening ceremonies and lecture starting Thursday, November 5th; followed by the full meeting beginning the next morning, Friday November 6th.
Read ArticleRheumNow Podcast – ACR 20 is Coming to Town (10.30.20)
Dr Jack Cush overviews the RheumNow commitment, upcoming bonus coverage of ACR20 and the good news and journal reports from the past week on RheumNow.com
Read ArticleAbatacept in Early Systemic Sclerosis (ASSET study)
An open-label extension of the ASSET trial (abatacept in diffuse cutaneous systemic sclerosis) showed continued safety with significant improvements in the modified Rodnan Skin Score (mRSS) at 12 months, suggesting abatacept (ABA) may benefit early systemic sclerosis (SSc).
Read ArticleFDA Invites Patient Input on Systemic Sclerosis Drug Development
While there have been several promising new agents for systemic sclerosis, many have failed - with all noting the difficulties in appropriate outcomes and endpoints for clinical trials. With this in mind, the FDA was interested in hearing patients’ perspectives on systemic sclerosis and new drug development, and held a virtual meeting on 10/13/20.
Read ArticleGuselkumab, an IL-23 Inhibitor, Effective in Psoriatic Arthritis
The DISCOVER‐2 trial has shown that guselkumab, an interleukin‐23p19 was shown to be safe and effective in biologic‐naïve psoriatic arthritis (PsA) patients in a one year study.
Read ArticleFibromyalgia Therapies Show Limited Evidence of Pain and QOL Reduction
JAMA Internal Medicine has published a review showing that most currently approved therapies for fibromyalgia (FM) do not have high-quality evidence to support their use; with some reducing pain or improving quality of life (QOL) in the short term, but overall effect size is not impressive.
Read ArticleVedolizumab Promising in Immune-Related GI Events
Vedolizumab (Entyvio) was superior to infliximab (Remicade) for treating immune-mediated diarrhea and colitis (IMDC), a researcher reported.
Read ArticleTelemedicine Outcomes in RA Care
A new short‐term follow‐up study has shown no significant difference in outcomes and quality measures in patients with RA who were managed by telemedicine (compared to usual in-person care).
Read ArticleFDA Hearing on COVID-19 Vaccines
Yesterday the Food and Drug Administration's (FDA) "Vaccines and Related Biological Products Advisory Committee" conve
Read ArticleRheumNow Podcast – Back Talk: Questions from Listeners (10.23.20)
Dr. Jack Cush reviews the news, journal reports, survey results and "Back Talk" questions from Rheums.
Read Article