All News
TNR Grand Rounds - Update on Biosimilars
This week's Tuesday Nite Rheumatology featured Dr Jonathan Kay (U Mass) who gave an update on Biosimilars and took questions the subject. The program was moderated by Dr. Jack Cush.
Read ArticleFDA Approves Taltz for Non-Radiographic Axial Spondyloarthritis
Today Eli Lilly announced that Taltz (ixekizumab) has received FDA approval for the treatment of adults with active non-radiographic axial spondyloarthritis (nr-axSpA).
Read ArticleFirst Look at COVID-19 Global Rheumatology Alliance Registry
Gianfresco et al have published the first peer-reviewed analysis of COVID-19 infected, rheumatic disease patients entered into the Rheumatology Global Alliance registry; showing that a) rheumatic disease patient can be infected with COVID-19, b) that DMARD and biologic use has no apparent effect on outcomes and c) steroid increase and TNF inhibitor decrease the odds of hospitalization.
Read ArticleRheumNow Podcast - Rheumatic Patients with COVID (5.29.20)
Dr. Jack Cush reviews the news and journal articles from the past week on RheumNow.com.
Read ArticleTargeting the Wnt Pathway Fails in OA
The Wnt pathway modulator lorecivivint failed to meet the primary endpoint in a phase IIa study for knee osteoarthritis (OA), but showed promise in certain patient subgroups, researchers reported.
Read ArticleTNR Grand Rounds - Cytokine Storm Syndromes
This week's Tuesday Nite Rheumatology featured Dr Randy Cron from the University of Alabama - Birmingham as he spoke and took questions on the immunology, immunopathogenesis and treatment of Cytokine Storm Syndrome, especially as they relate to COVID-19 and rheumatic disease patients. The program was moderated by Dr. Jack Cush.
Read ArticleBoston and Wuhan Report Rheum COVID Patients at Risk for Respiratory Failure
Two current reports from Boston and Wuhan describe cohorts of COVID-19 (+) rheumatic disease patients who generally do well but appear to have a higher risk of pulmonary involvement.
Read ArticleHydroxychloroquine Improves Survival in Lupus
Hydroxychloroquine (HCQ) has dominated the news in recent weeks, because of poor outcomes reported when used in COVID patients. Yet, a recent report reaffirms what rheumatologists have known for years - that HCQ use is associated with better outcomes in systemic lupus erythematosus, including survival.
Read ArticleLow Risk of COVID-19 Pneumonia in Rheumatic Patients
A current letter in the Annals of Rheumatic Disease details the rheumatic disease patient cohort outcomes from the University of Siena, Italy showing only 2 cases of COVID-19 among 859 patients treated with tsDMARDs and bDMARDs.
Read ArticleAgain, Antimalarial Use Fails to Benefit COVID
Today Lancet reported the results of another retrospective trial showing that hydroxychloroquine or chloroquine, alone or with a macrolide antibioitic, offered no additional benefits to COVID-19 patients, but was associated with higher rates of mortality and arrhythmia.
Read ArticleNo Certain Link between Biologics and Melanoma Risk
A review of available data fails to show an increased risk of melanoma in IMID (inflammatory bowel disease, rheumatoid arthritis, and psoriasis) patients treated with systemic biologic therapies.
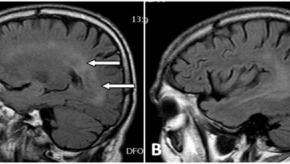
Read ArticleAnti-TNF and CNS Events: The Link Strengthens
Patients with autoimmune diseases who were treated with tumor necrosis factor (TNF) inhibitors had an increased risk of developing inflammatory central nervous system (CNS) adverse events, a nested case-control study found.
Read ArticleTNR - COVID-Rheumatology Registry & COVID in OZ
This week's Tuesday Nite Rheumatology features presentations and interviews with Drs. Peter Nash and Philip Robinson. Dr. Robinson presents latest data on the Rheum-Covid.org Registry, that he helped establish under the Global Rheumatology Alliance. Dr. Nash presents the impact of COVID in Australia and pragmatic COVID management.
Read ArticleSystemic Sclerosis Future Therapies and Outcome Measures
Nagaraja, Khanna and colleagues have published an overview of current and future therapies in patients with systemic sclerosis (SSc) and have reviewed the potential outcome measures for this difficult autoimmune disorder.
Read ArticleHigh Dose Anakinra Effective in COVID-19
Anakinra was studied in 29 COVID(+) patients with respiratory distress and high inflammatory markers (CRP or ferritin) and compared to non-anakinra patients, those on anakinra had better survival (90% vs. 56% ;p=0·009) and greater improvements in CRP and pulmonary function (72% vs 50%) compared to controls at day 21.
Read ArticleThe Nine Lives of Hydroxychloroquine (Updated)
Hydroxychloroquine is one of many medications frequently used in rheumatology practice. Its remarkable versatility is attested by its routine use in lupus, in patients with an autoimmune coagulopathy, in patients with rheumatoid arthritis, as well as in those with a low-level inflammatory arthropathy.
Read ArticleRheumNow Podcast - COVID Kids and Men (5.15.20)
Dr. Jack Cush reviews the news and journal articles from the past week on RheumNow.com.
Read ArticleCDC on Pediatric Multi-System Inflammatory Syndrome
The latest hazardous spinoff to the coronavirus infection is an inflammatory, Kawasaki-like syndrome unique to children or adolescents with COVID-19. Sporadic reports from around the globe of this severe pediatric COVID syndrome have littered the news with brief mentions and little detail.
Read ArticleKids' COVID-Linked Ailment Is Not Your Typical Kawasaki Disease
As data continue to emerge about a multi-system inflammatory disorder in children apparently connected to COVID-19, evidence is growing that this is not your typical Kawasaki disease.
Read ArticlePlaquenil Does Not Protect Lupus Patients from COVID-19
Annals of Rheumatic Disease reports on an analysis of lupus (SLE) patients that shows COVID-19 infection rates were similar between those lupus patients who were taking hydroxychloroquine (HCQ) and those not taking HCQ.
Read Article