Sjögren's disease RCTs: Has the Long Drought Ended? Save
Sjögren's disease has been a difficult space for new FDA approvals. TNF inhibitors, rituximab, tocilizumab, and our favorite connective-tissue-drug hydroxychloroquine have all been studied in RCTs, all of which failed to meet their primary endpoints. The typical litany of problems confronting trials in connective tissue diseases have been offered as explanation; the endpoints are complicated, the patients are heterogenous, and the primary symptoms arise from a motley collection of inflammation, damage, and concurrent fibromyalgia.
At ACR 2025, two late breaking abstracts have triggered hope for patients suffering from Sjögren's disease.
The first studied telitacicept, an antibody designed to inhibit both BAFF and APRIL (think belimumab + some APRIL, which will also downregulate antibody-forming plasma cells). LB11 reported headline results from a phase 3 study of nearly 400 patients from China. The study met its primary endpoint of EULAR Sjögren’s Syndrome Disease Activity Index (ESSDAI) response at week 24.
Like me, you probably have no idea what’s in the ESSDAI. In short, it scores patients over 12 domains that are relevant to Sjögren's disease. The study of telaticicept observed an impressive 4-point difference between placebo and the 160mg telitacicept group. This difference is not only impressive, but also peculiar. The difference between groups emerged because there was essentially no placebo response, which would be highly surprising. I am cautiously optimistic about this therapy, with a strong emphasis on caution and a stronger requirement for confirmatory trials.
The second therapy of note for Sjögren's disease is ianalumab, a BAFF-R drug that that met it’s primary endpoint (change from baseline in ESSDAI) in two large phase 3 RCTS (NEPTUNUS-1 and NEPTUNUS-2) (LB24). Especially among those with higher salivary flow rates at baseline (ie more tissue to save), they also observed an improvement in stimulated salivary flow rates. Thankfully this came at a relatively low cost from a safety perspective – infectious complications were on the low end for the B cell depleting space. The pooled analysis even identified an improvement in patient global assessment.
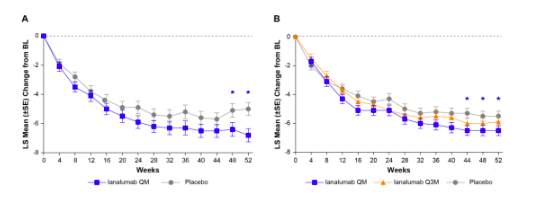
Figure 1: Primary endpoints from NEPTUNUS 1 and NEPTUNUS 2 from ACR 2025. Note the large placebo response, which more closely accords what I would have expected from the telitacicept trial
As usual, the plot thickens when you dig deer pinto the data. First, I am not a big fan of the ESSDAI. It does not include fatigue, it under-emphasizes joint pains, and the “biological domain” means relatively unimportant laboratory changes can drive outcomes. We do not yet know if it positive affected joint pains, but the FACIT-Fatigue scores that were reported during the oral session did not differ between the treatment and placebo groups.
Second, “statistical significance” is not synonymous with “clinically meaningful.” This is an inexact science, but “minimally clinically important differences” (MCID) can be used to define what score on some metric would matter to a patient. Most people would agree that an MCID above 3 for the ESSDAI and above 10 for the patient global would be adequate. Unfortunately, the aggregate values fell below those thresholds for ianalumab.
Critiques notwithstanding, it’s exciting to see progress in Sjögren's disease. I will be looking forward to a replication study of telitacicept (which I think is necessary) and the publication of the NEPTUNUS studies (probably coming to NEJM someday soon). I would be optimistic about an FDA approval for ianalumab and am hoping the patient populations most likely to benefit will be more clear as we see more data.










If you are a health practitioner, you may Login/Register to comment.
Due to the nature of these comment forums, only health practitioners are allowed to comment at this time.