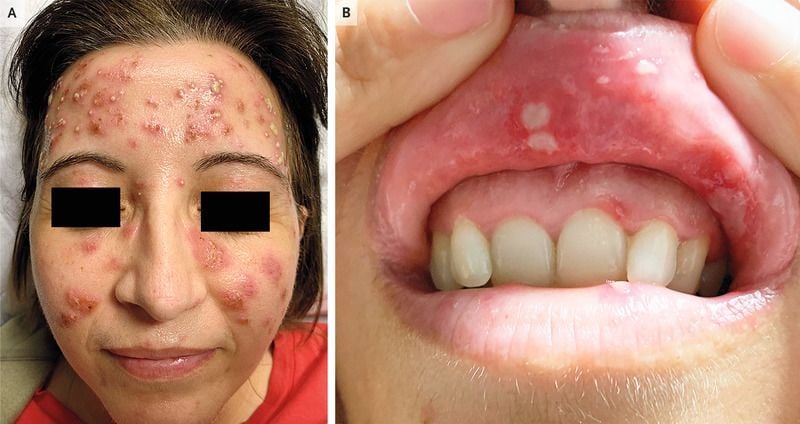
FDA has granted Soligenix "Orphan Drug Designation" to study dusquetide in the treatment of Behçet's dz, based on an 8 patient proof of concept Phase 2a trial. Not yet FDA approved, dusquetide is a short peptide innate defense regulator (IDR). Behcets affects 18K in USA and https://t.co/RzzVuQ6bAB
Links:
Aug-23-2025



