Obinutuzumab Induces Histologic Remission and Deep Kidney Parenchymal B-Cell Depletion in Participants With Lupus Nephritis: Exploratory Analyses of the Phase III REGENCY Trial
Brad H. Rovin,1 Elsa Martins,2 Cary D. Austin,3 Harini Raghu,3 Caleb Chan,3 Patrick S. Chang,3 Jay P. Garg,3 Valeria Alberton,4 Mittermayer B. Santiago,5 Gustavo Aroca-Martínez,6 Fedra I. Palazuelos,7 Teresa Baczkowska,8 Jose Alfaro,9 Jorge Ravelo-Hernández,10 Richard A. Furie,11 Luis F. Pinto,12 Eduardo H. Albiero,13 Christopher Larsen,14 Bongin Yoo,3 Jennifer Pulley,15 Andrew Thorley,3 Thomas Schindler,2 Theodore A. Omachi,3 William F. Pendergraft,3Ana Malvar16
1Department of Internal Medicine, The Ohio State University College of Medicine, Columbus, OH, USA; 2 F. Hoffmann-La Roche Ltd, Basel, Switzerland; 3 Genentech, Inc., South San Francisco, CA, USA; 4 Pathology Unit, Fernandez Hospital, Buenos Aires, Argentina; 5 Bahiana School of Medicine and Public Health and UFBA, Federal University of Bahia, and Clínica SER da Bahia, Salvador, Brazil; 6 Universidad Simon Bolivar, Barranquilla, Colombia y Clínica de la Costa, Barranquilla, Colombia; 7 Centro de Investigación y Tratamiento Reumatológico S.C., Mexico City, Mexico; 8 Department of Transplantation Medicine, Nephrology and Internal Medicine, Medical University of Warsaw, Warsaw, Poland; 9 Instituto Peruano del Hueso y la Articulación, Lima, Peru; 10Clinica San Juan Bautista, Unidad de Investigacion en Reumatologia e Inmunologia, Lima, Peru; 11Division of Rheumatology, Northwell Health, Great Neck, NY, USA; 12Internal Medicine and Rheumatology, Hospital Pablo Tobón Uribe, Medellín, Colombia; 13Sanatorio Allende, Córdoba, Argentina; 14Arkana Laboratories, Little Rock, AK, USA; 15Roche Products Ltd, Welwyn Garden City, UK; 16Organización Médica de Investigación, Buenos Aires, Argentina
Disclosures:
B.H.R. has received consulting fees from F. Hoffmann-La Roche Ltd/Genentech Inc.
E.M. and T.S. are employees and shareholders of F. Hoffmann-La Roche Ltd.
C.D.A., H.R., C.C., P.S.C., J.P.G., B.Y., A.T., T.A.O. and W.F.P. are employees of Genentech, Inc. and shareholders of F. Hoffmann-La Roche Ltd.
V.A. has received consulting fees and/or reported professional services for F. Hoffmann-La Roche Ltd, GlaxoSmithKline and Novartis.
M.B.S., G.A., T.B., L.F.P. and E.H.A. have nothing to disclose.
F.I.P. has received consulting fees and/or research support from AbbVie, Amgen, Eli Lilly, F. Hoffmann-La Roche Ltd, Janssen, Novartis, Pfizer and Takeda.
J.A. has received consulting fees and/or reports professional services for AstraZeneca, Bristol Myers Squibb, F. Hoffmann-La Roche Ltd, Horizon Therapeutics and Kezar.
J.R. has received consulting fees and/or reported professional services for AstraZeneca, Bristol Myers Squibb, F. Hoffmann-La Roche Ltd and Horizon Therapeutics.
R.A.F. has received research support and consulting fees from Chugai Pharmaceutical Co., Ltd., F. Hoffmann-La Roche Ltd, Genentech, Inc. and GlaxoSmithKline; is a consultant for AstraZeneca, EMD Serono, Novartis, Biogen, and BMS; is an investigator for AstraZeneca, Novartis, Biogen, BMS, and Kyverna; and is a speaker for AstraZeneca.
C.L. has received support and/or consulting fees from Calliditas Therapeutics AB and Novartis.
A.M. has received consulting fees and/or reports professional services for Bristol Myers Squibb,
F. Hoffmann-La Roche Ltd, GlaxoSmithKline, Kezar, Novartis and Pfizer.
Background
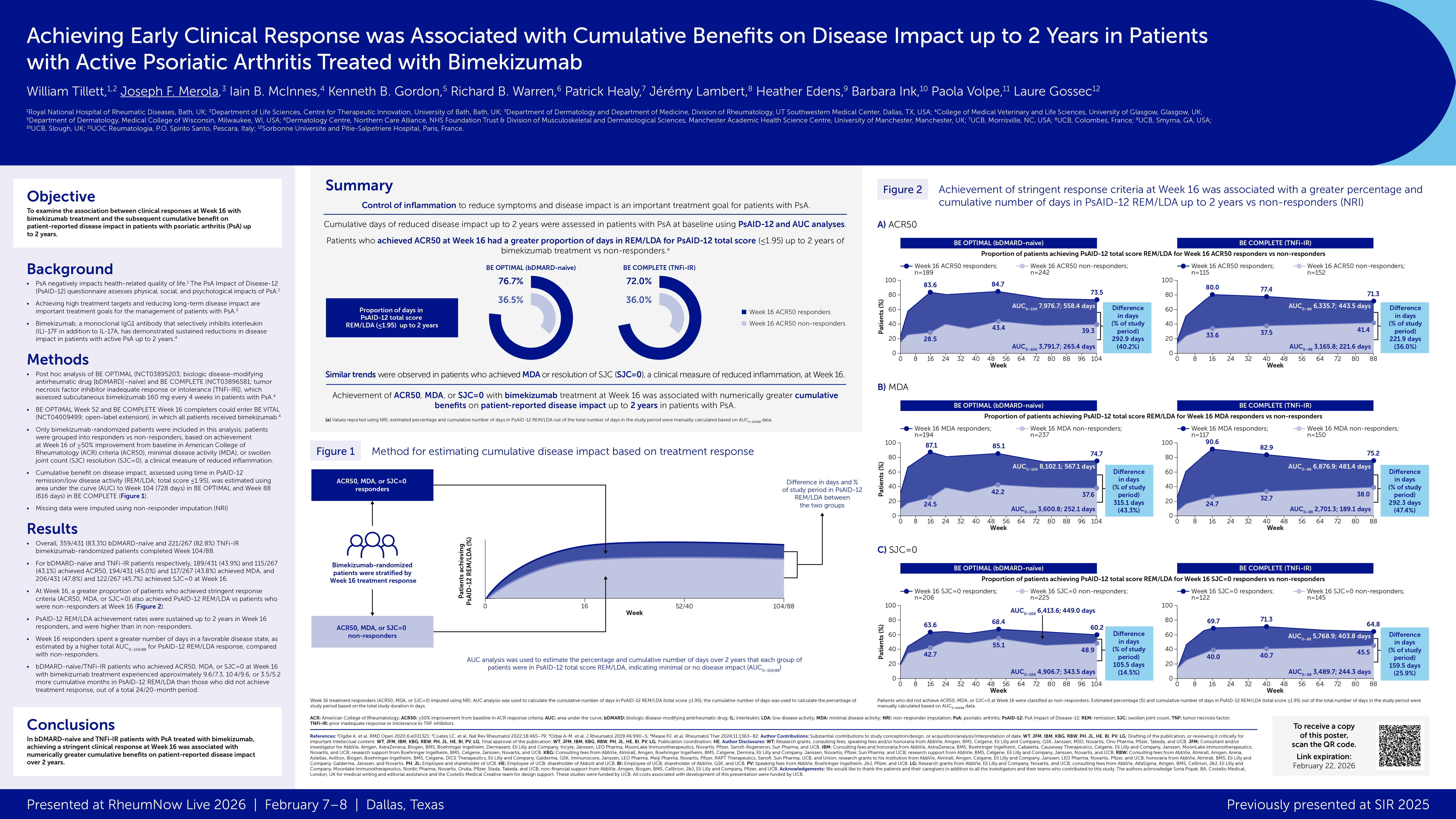
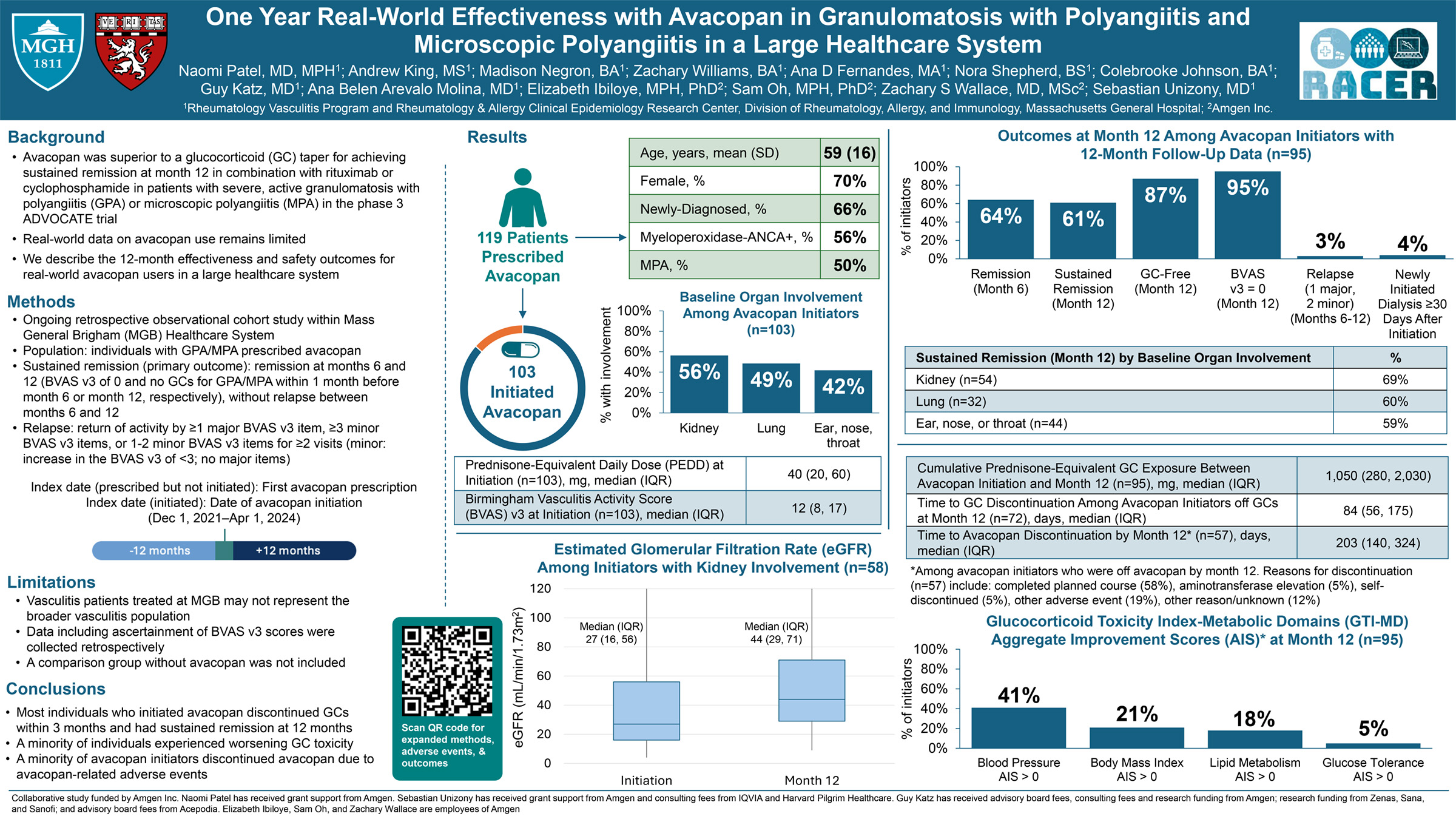
The REGENCY trial (NCT04221477) demonstrated superiority of obinutuzumab (OBI) plus standard therapy (+ST) vs placebo (PBO) +ST in achieving complete renal response (CRR) at Week 76 (W76) in adults with active lupus nephritis (LN). These exploratory analyses aimed to evaluate histologic remission and kidney tissue-level B-cell depletion at W76 in participants treated with OBI+ST vs PBO+ST.
Methods
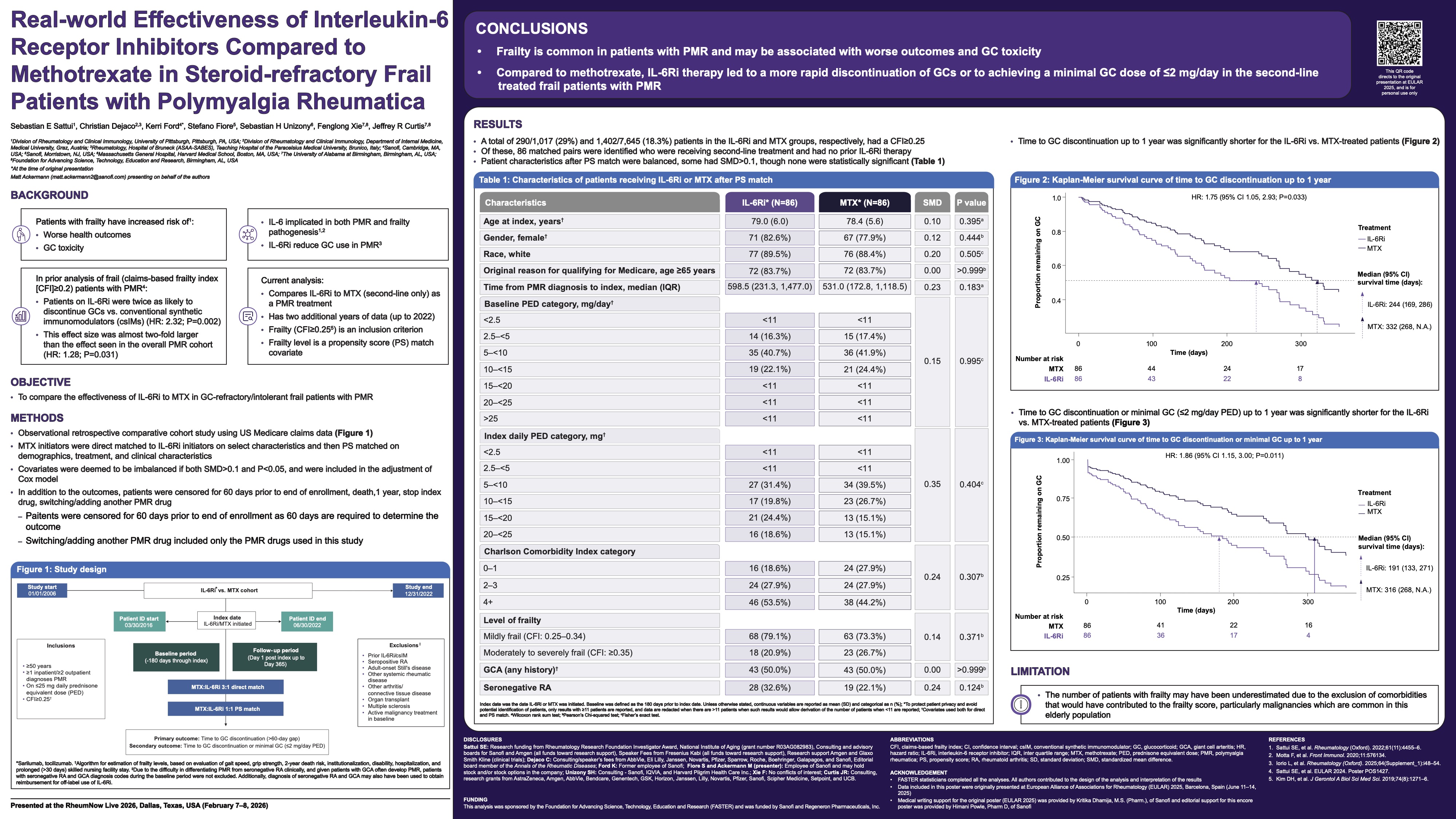
Paired baseline and W76 kidney biopsies were analyzed. Histologic analysis: 64 biopsies (32 OBI+ST, 32 PBO+ST) were evaluated using the 2018 ISN/RPS LN classification, along with the NIH activity (AI) and chronicity indices. The proportion of participants achieving histologic or near-histologic remission (AI=0 or ≤1) was determined. B-cell analysis: 29 participants (14 OBI+ST, 15 PBO+ST) were assessed. CD79a+/CD138− B cells were quantified by
immunofluorescence microscopy and digital whole-slide analysis. Changes in B-cell counts at W76 were compared using an ANCOVA model, adjusting for baseline B-cell counts and stratification factors.
Results
Baseline characteristics were balanced, despite higher tissue B-cell levels in the OBI+ST group. At W76, significantly more participants achieved AI=0 or ≤1 with OBI+ST vs PBO+ST. Among participants not achieving CRR, 52.6% (10/19) in the OBI+ST group had an AI=0 at W76 vs 8.3% (2/24) in the PBO+ST group. Almost every participant in the OBI+ST group had a drop in their tissue B-cell count by W76. Adjusted mean change in B-cell counts from baseline to W76 was −28.5 (95% CI, −33.3 to −23.6) for OBI+ST vs −11.9 (95% CI, −16.6 to −7.2) for PBO+ST, a significant difference of −16.6 (95% CI, −23.4 to −9.7; P<0.0001).
Conclusions
In the largest longitudinal kidney biopsy cohort ever reported for a registrational LN clinical trial, significantly more participants achieved complete/near-complete histologic remission with OBI+ST vs PBO+ST. Obinutuzumab’s potent B-cell clearance from kidney tissue may drive kidney function improvement and LN flare reduction.
Additional abstract information
Acknowledgements: We thank all study participants, the staff and the study investigators. This study was funded by F. Hoffmann-La Roche Ltd. Editorial assistance was provided by Nucleus Global and funded by F. Hoffmann-La Roche Ltd.