All News
ANA Pollution (2.06.2026)
Dr. Jack Cush reviews the news, journal articles and regulatory news from this past week on RheumNow.com
Read ArticleRheumNow Live Preview (1.30.2026)
Dr. Jack Cush reviews the news and journal reports from RheumNow.com - and the top 5 reasons to attend RheumNow Live 2026, which is one week away.
Read ArticleB Cells at the Brink in Sjögren Disease
The current issue of Arthritis & Rheumatology reviews current understanding of the immunopathogenesis of Sjögren disease (SjD) and how that has influenced the quest for drug development for this most systemic autoimmune disorder.
Read Article
FDA sent a complete response letter to AstraZeneca on their application (BLA) for anifrolumabs (Saphnelo) subcutaneous use in SLE. Despite a positive TULIP-SC trial & EU approval of SC-anifrolumab, FDA & sponsor still have to work things out. CRL reasons are unknown https://t.co/3dNwEyolrj
Dr. John Cush RheumNow ( View Tweet)
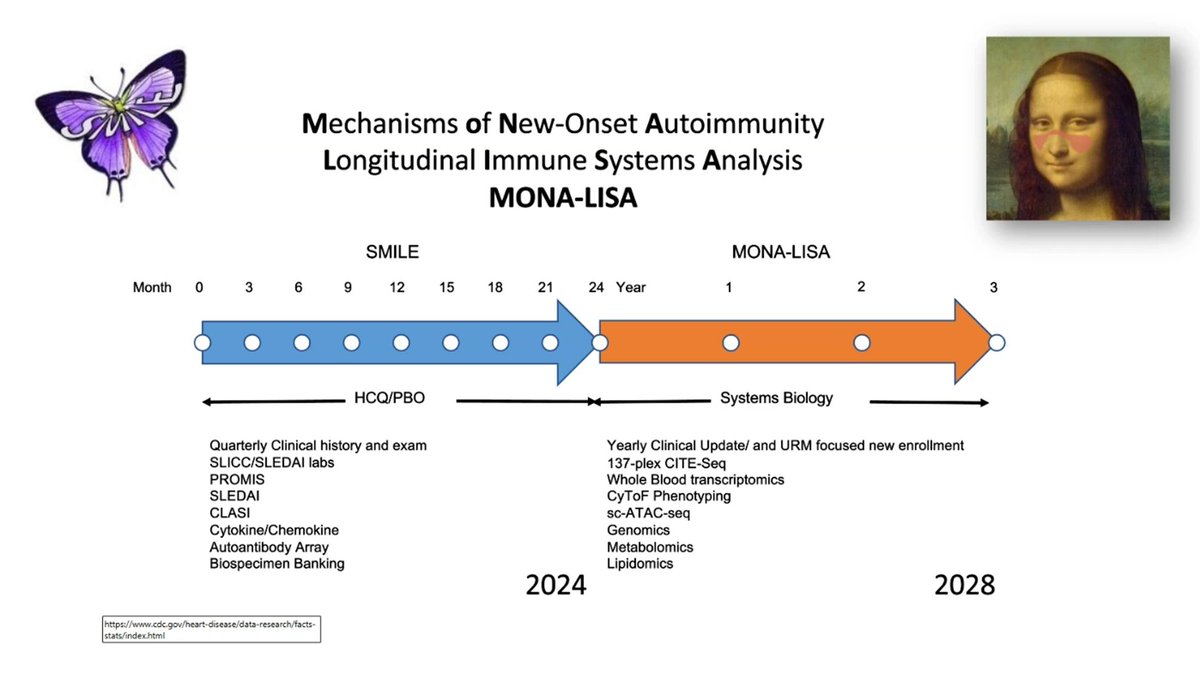
NEXT STEPS: Karp and investigators are engaged in the MONA LISA study - Using smile Data and biospeciments – they intend to look for biomarkers that may better predict the future development of SLE Dr. David Karp lecturing at UTSW @drdavidkarp @utswrheum https://t.co/acZD1kmNhq
Dr. John Cush RheumNow ( View Tweet)
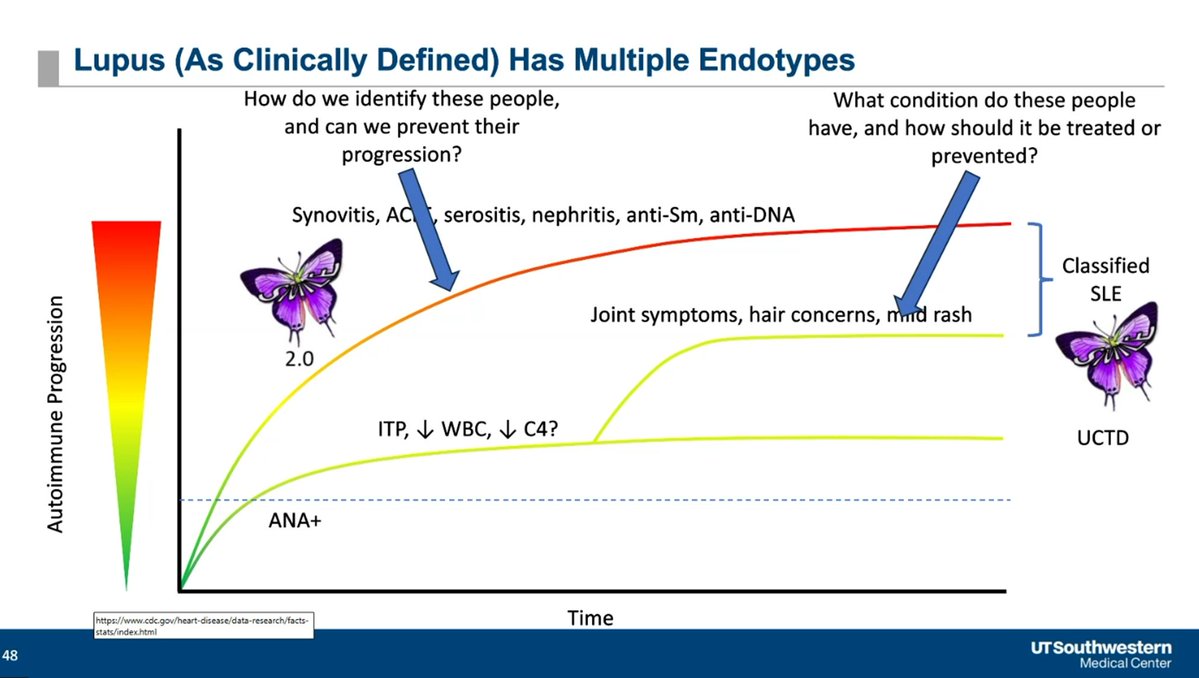
The challenge may be that lupus has multiple endotypes with variable rates of progression/trajectories. Can you Prevent SLE? Dr. David Karp lecturing at UTSW @drdavidkarp @utswrheum https://t.co/BA4KRKebIv
Dr. John Cush RheumNow ( View Tweet)
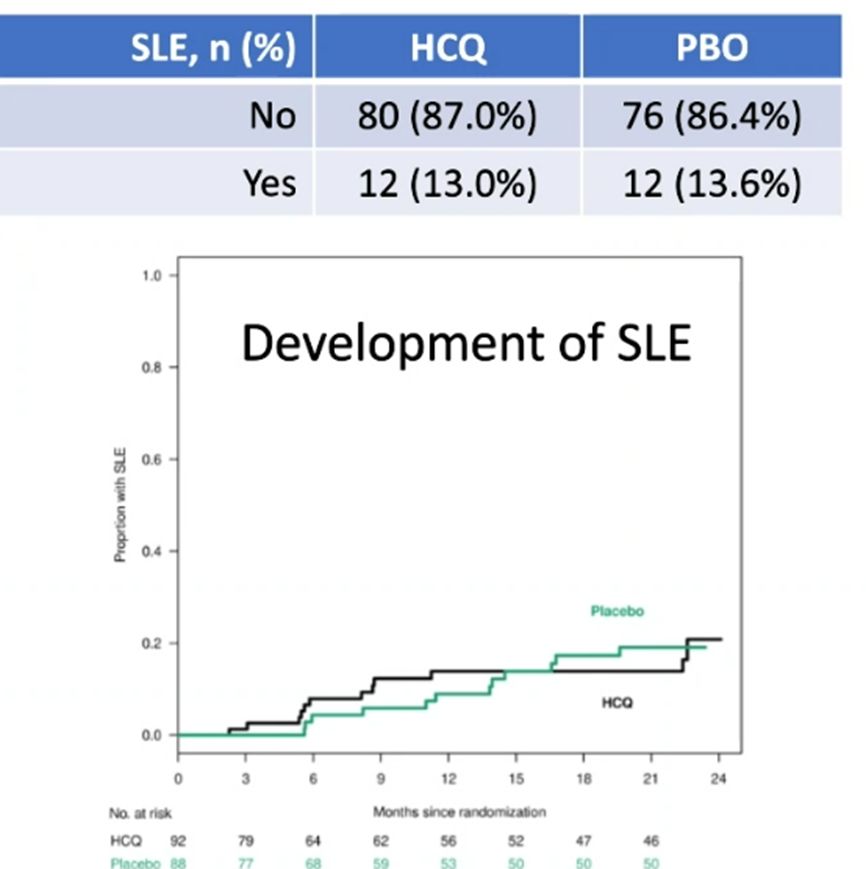
SMILE enrollment hampered by COVID - yet failed to show a protective effect for HCQ in preventing SLE. Can you Prevent SLE? Dr. David Karp lecturing at UTSW @drdavidkarp @utswrheum https://t.co/GImHpycbgO
Dr. John Cush RheumNow ( View Tweet)
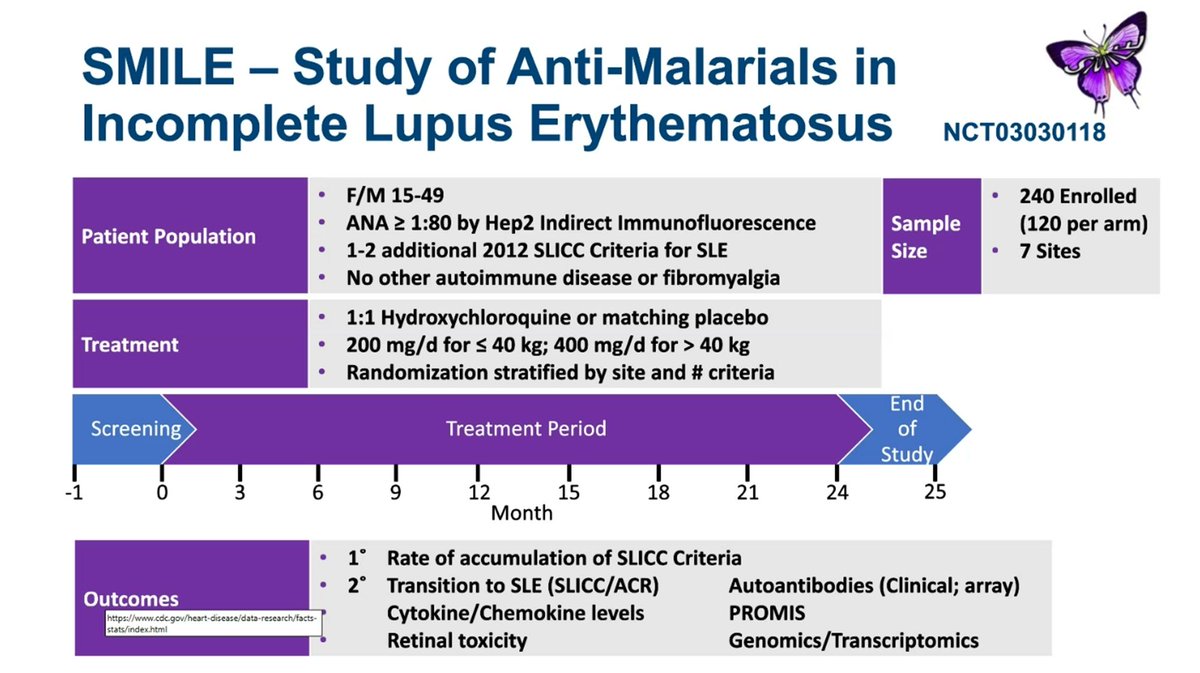
REcently published SMILE study looked at the potential of HCQ (vs PBO) to prevent SLE in at risk people. Had to be ANA+ and 1-2 SLICC criteria at entry. Can you Prevent SLE? Dr. David Karp lecturing at UTSW @drdavidkarp @utswrheum https://t.co/4FY0kOLdRT
Dr. John Cush RheumNow ( View Tweet)
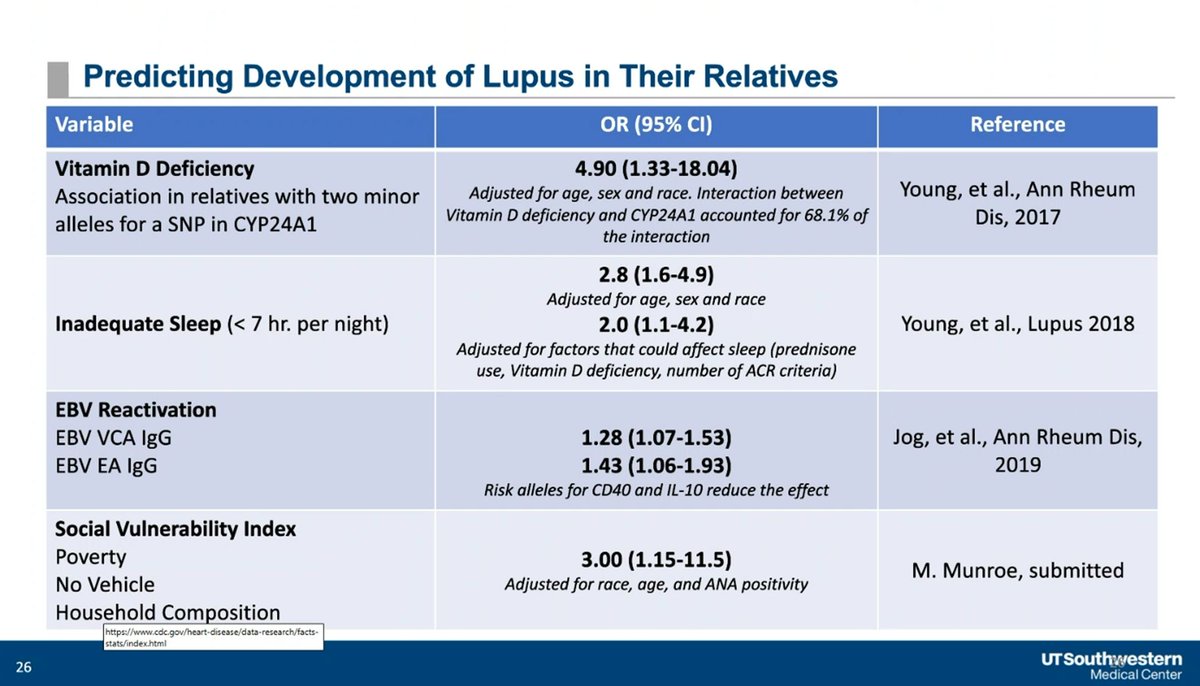
Factors that may augment future development of SLE - includes Vitamin D levels, poor sleep, EBV reactivation & social determinants of health. Can you Prevent SLE? Dr. David Karp lecturing at UTSW @drdavidkarp @utswrheum https://t.co/e3TKATcxXB
Dr. John Cush RheumNow ( View Tweet)
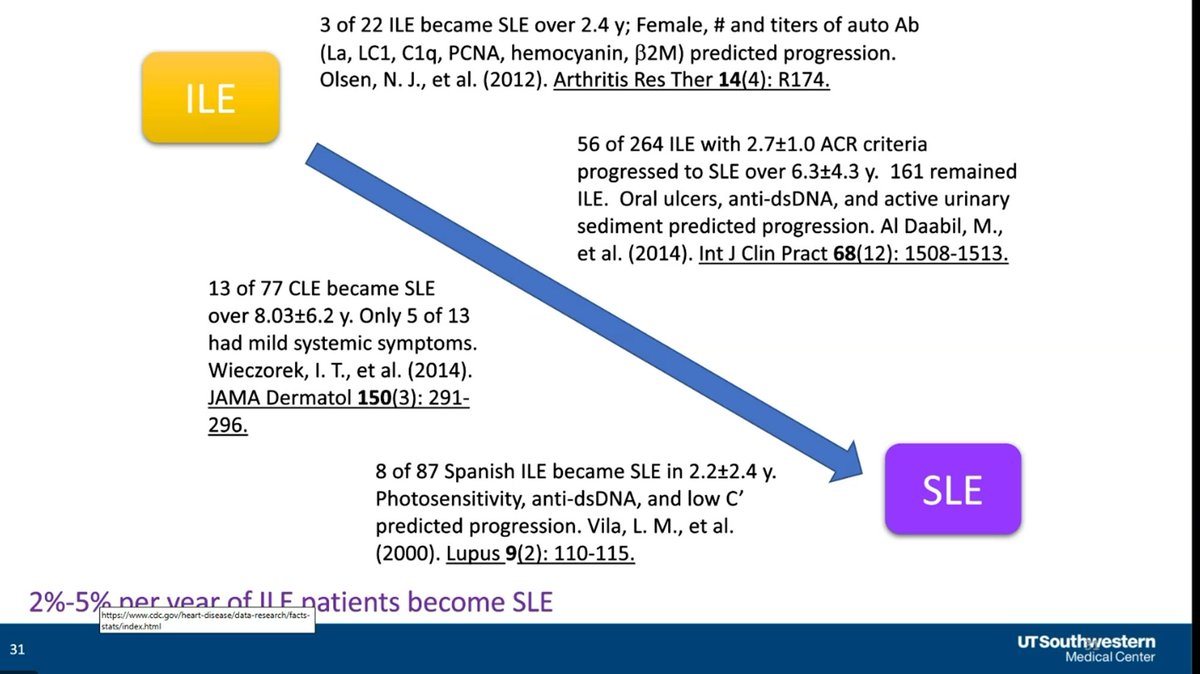
Only a subset of incomplete lupus (ILE) will progress to SLE - betw 1 in 10 and 1 in 5. Can you Prevent SLE? Dr. David Karp lecturing at UTSW @drdavidkarp @utswrheum https://t.co/oo40Dumk4I
Dr. John Cush RheumNow ( View Tweet)
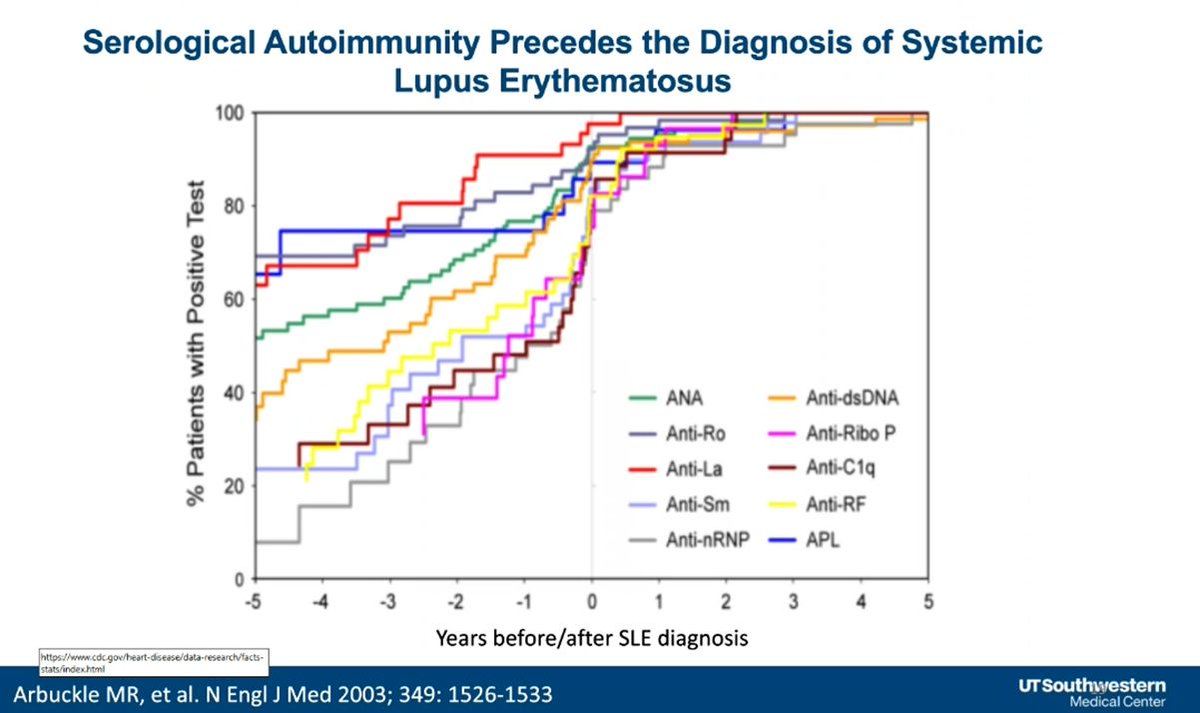
Can you Prevent SLE? Dr. David Karp lecturing at UTSW Showing that autoantibodies exist long before the onset of SLE @drdavidkarp @utswrheum https://t.co/zPAYbSlIBS
Dr. John Cush RheumNow ( View Tweet)

FDA has announced "Breakthrough Designation" for 3 drugs + 1 test being developed:
** Biogen: litifilimab (targets dendritic cells) for cutaneous lupus
** Novartis: Ianalumab (BAFF blockade) for Sjogrens
** J&J: Nipocaiimab (FcRn) for Sjogrens
** Encarta: pocket Lyme test https://t.co/zODOTWyqP8
Dr. John Cush RheumNow ( View Tweet)

Roche Canada announced that Health Canada has approved obinutuzumab (Gazyva) for the treatment of adult patients with active lupus nephritis who are receiving standard therapy. Following four initial doses in the first year, Gazyva can be administered twice yearly https://t.co/P4dCVkoPCH
Dr. John Cush RheumNow ( View Tweet)

Remission? Single center study of 100 RA, 100 SLE pts assessed by SLEDAI-2K, SDAI, VAS. Remission/LDA found in 88% of 392 SLE visits & 91% of 389 RA visits(p=NS). Remission was more common in SLE vs RA (79% vs 69%; p= 0.0018). https://t.co/ZPRrYQMsCT
Dr. John Cush RheumNow ( View Tweet)

Japanese nationwide survey of 1943 RMD pts taking Azathioprine (AZA) 34% had adverse events: hepatobiliary (149%), GI (10%), blood/lymphatic (9%), infections (5%), & skin (2.4%). AEs lead to 71% D/C AZA. Serious AEs (Gr ≥3) higher w/ older (OR 2.5), & SLE (OR 2.3) https://t.co/lVNYVQATfx
Dr. John Cush RheumNow ( View Tweet)

Lupus Foundation announced today that US House of Representatives have approved $27 Million in Funding for Lupus Programs in 2026 w/ fund available from (CDC), OMH, & DoD. Funding increased 40% @ CDC, 33% @OMH, & $415 million @ National Institutes of Health https://t.co/ENcykcW7yA
Dr. John Cush RheumNow ( View Tweet)

Roche Canada announced that Health Canada has approved obinutuzumab (Gazyva) for the treatment of adult patients with active lupus nephritis who are receiving standard therapy. Following four initial doses in the first year, Gazyva can be administered twice yearly https://t.co/snAVErShjK
Dr. John Cush RheumNow ( View Tweet)
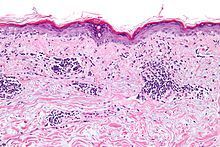
Lupus Accelerating Breakthroughs Consortium commissioned a stakeholders group (including the FDA) to assess drug development in Cutaneous lupus CLE), and they have endorsed CLASI (CLE Dz Area & Severity Index) as the outcome measure for CLE clinical trials. https://t.co/q7If97AHBa
Dr. John Cush RheumNow ( View Tweet)
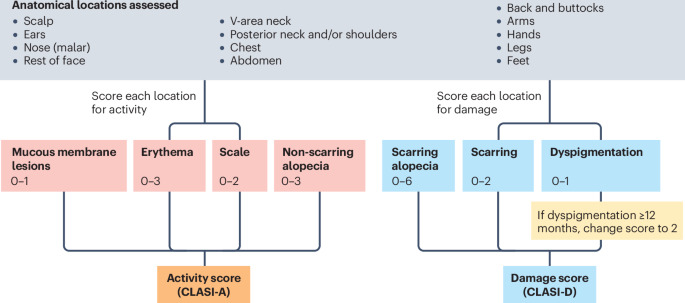
New content online: Recommendations for the use of CLASI as an outcome measure in cutaneous lupus erythematosus clinical trials https://t.co/nDxfWWgutC https://t.co/Z6JMojdBz4
Links:
NatRevRheumatol NatRevRheumatol ( View Tweet)

Subcutaneous Anifrolumab in SLE
Manzi et al. have published the results of the TULIP-SC trial that showed that weekly subcutaneous (SC) anifrolumab, when given to severe SLE patients, had comparable efficacy and safety to the approved intravenous (IV) anifrolumab. https://t.co/EzaqS5q096
Dr. John Cush RheumNow ( View Tweet)