All News
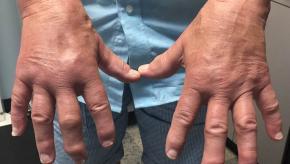
Patients Prefer Nurse Led Care for their Gout
Despite the well known, well publicized treat-to-target (T2T) goal of a serum uric acid (SUA) level 6 mg/dl, this goal is seldom achieved ( 40%) in clinical practice and patient adherence has been unacceptably low. A recent study shows that nurse-led care led to better outcomes in gout including patient acceptability, long-term adherence, and less flares.
Read ArticleVoluntary Recall of Zantac by Sanofi/FDA
Sanofi has initiated a voluntary recall of all Zantac OTC (over-the-counter) products in the United States. This includes Zantac 150®, Zantac 150® Cool Mint, and Zantac 75®.
Read ArticleFDA Approves Ustekinumab for Ulcerative Colitis
Ustekinumab (Stelara) is now FDA-approved to treat moderately to severely active ulcerative colitis, said drugmaker Janssen on Monday, in addition to its indications for psoriasis, psoriatic arthritis, and Crohn's disease.
Read ArticlePain Persists Despite TNF Inhibitor Use
Control of pain in patients with rheumatoid arthritis (RA) often focuses on control of inflammation as a means to better control pain.
Read ArticleAdalimumab and Pregnancy Outcomes
A prospective study of birth outcomes to mothers exposed to adalimumab (ADA) between 2004 and 2016 was conducted by Organization of Teratology Information Specialists (OTIS) Research Center at the University of California San Diego, and showed that ADA exposure was not associated with an increase
Read ArticleInterleukin Targeted Biologics Increase Risks of Infection
A systematic review of the literature shows that, compared to placebo, the use of non-TNF, interleukin inhibitor biologics may be associated with significantly higher rates of serious infections, opportunistic infections, and cancer.
Read ArticleRheumNow Podcast – Fight or Switching (DMARDs) (10.18.19)
Dr. Jack Cush reviews the news and journal articles from the past week on RheumNow.com.
Read ArticleStress and the Risk of Incident Inflammatory Arthritis
A prospective analysis of newly diagnosed, inflammatory arthritis (IA) patients suggests that perceived distress (stress) increases the odds of incident IA.
Read ArticleLong Delays for Inflammatory Arthritis Patients
The National Rheumatoid Arthritis Society's (NRAS) annual audit has identified significant treatment delays for patients with suspected early inflammatory arthritis could result in unnecessary harm.
Read ArticleSprifermin Benefits Cartilage Loss but not Symptoms in Knee Osteoarthritis
Intra-articular sprifermin given to patients with symptomatic and radiographic knee osteoarthritis has been shown to significantly improve total femorotibial joint cartilage thickness after 2 years, but without significant clinical benefits. Which begs the question, why is there a disconnect between radiographic disease modification (cartilage thickness) and symptomatic improvement?
Read ArticleBisphosphonates and the Risk of Osteonecrosis of the Jaw
Even though oral bisphosphonates are widely used, there is an inordinate concern over the risk of osteonecrosis of the jaw (ONJ). A new UK study suggests that the risk of ONJ is elevated six fold by the use of biphosphonates.
Read ArticleBiologics Lead the Way in Drug Price Increases
Reuters has reported the results of a recent Institute for Clinical and Economic Review (ICER) analysis showing that biologics, especially Humira and Rituxan, are leading the way in the cost of drugs in the USA.
All told, Humira and Rituxan topped a list of seven treatments whose combined 2017 and 2018 price hikes accounted for a $5.1 billion increase in U.S. drug spending. ICER said their analysis points to price hikes that were more than twice the rate of medical inflation and were not warranted by any new clinical evidence.
Serum Interferon Predicts Lupus Flares
Elevated serum levels of interferon-α among patients whose systemic lupus erythematosus (SLE) was in remission helped predict future disease flares, European researchers found.
Read ArticleFUTURE 5 - Secukinumab and Less Radiographic Progression in Psoriatic Arthritis
The FUTURE 5 trial studied the effect of secukinumab (SEC) on radiographic progression through 52 weeks in patients with active psoriatic arthritis (PsA) and found that SEC was clinically and radiographically superior to placebo (PBO).
Patients received s.c. secukinumab 300 mg load (300 mg), 150 mg load (150 mg), 150 mg no load regimens or placebo at baseline, at weeks 1, 2 and 3 and every 4 weeks starting at week 4. The majority (87%) of patients enrolled at baseline remained in the study for 52 weeks.
RheumNow Podcast – Women Take Over Rheumatology (10.4.19)
Dr. Jack Cush reviews the News and Journal Reports from this week on RheumNow.com.
Read ArticleASBMR Recommendations on Secondary Fracture Prevention
The American Society for Bone and Mineral Research has developed multistakeholder consensus clinical recommendations for the prevention of secondary fractures for those aged 65 years and older after an initial hip or vertebral fracture.
Read ArticleIxekizumab vs. Adalimumab in Psoriatic Arthritis
The Annals of Rheumatic Disease reports a psoriatic arthritis study where in ixekizumab was non-inferior to adalimumab for achievement of ACR50 responses but was superior to adalimumab for achievement of PASI100 by week 24.
Read ArticleMMWR: Increased Opioid Use in Lupus
Opioids are generally not indicated for pain in systemic lupus erythematosus (SLE) and other rheumatic diseases because of limited efficacy and risks for preference and adverse health effects.
Read ArticleLow Dose IL-2 Effective in Lupus
A double-blind, placebo-controlled clinical trial of low-dose IL-2 in patients with systemic lupus erythematosus (SLE) has shown that low-dose IL-2 induced was clinically effective while expandng regulatory T cells and NK cells, which may benefit immune homeostasis in SLE patients.
Arthritis Foundation Releases First CBD Guidance for Adults With Arthritis
As the leading organization for people with arthritis, the Arthritis Foundation has just released the first CBD guidance for adults with arthritis. CBD, or cannabidiol, a plant-based compound, has become popular among people with arthritis seeking to ease chronic joint pain.
Read Article