All News
RheumNow Live is Coming to Town (3.11.2022)
Dr. Jack Cush reviews the news and journal articles from the past week on RheumNow.com.
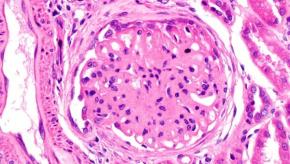
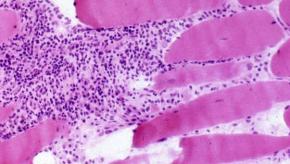
Read ArticleCould Anifrolumab Work in Lupus Nephritis?
Anifrolumab is effective and FDA approved for use in systemic lupus erythematosus (SLE); and now, a trial in lupus nephritis that almost shows benefit.
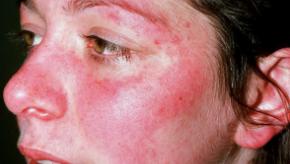
Read ArticleIncreased SLE Flares with Hydroxychloroquine Reduction
Concerns about the long-term safety of hydroxychloroquine (HCQ) often results in dose reduction or drug discontinuation; yet a current study shows that, for those in remission, HCQ taper/discontinuation resulted in a significantly higher rate of SLE flares.
Read ArticleDiagnostic Delay in Axial Spondyloarthritis (2.25.2022)
Dr. Jack Cush reviews the news and journal reports from the past week on RheumNow.com. This week, rheumatologic problems incited by respiratory infections, air pollution and how you live, now where you live.
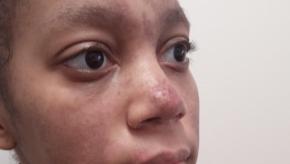
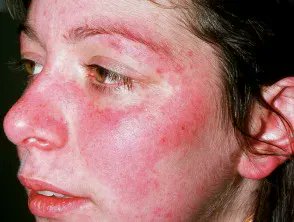
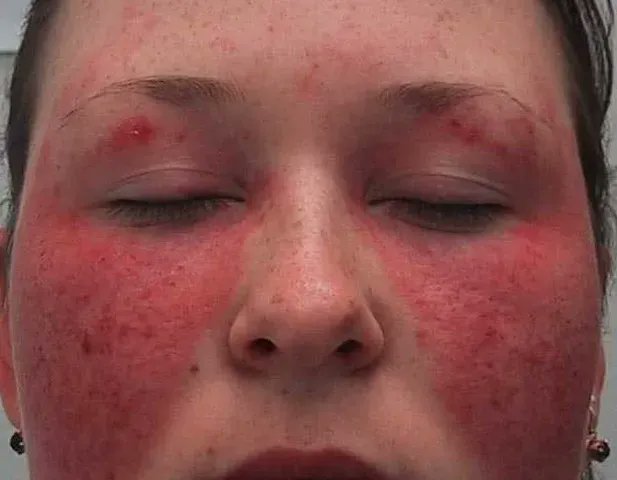
Read ArticleACR Addresses Racial Disparities in Lupus Trials
The American College of Rheumatology (ACR) is launching two new initiatives to reduce racial disparities in lupus clinical trials: Training to Increase Minority Enrollment in Lupus Clinical Trials with CommunitY Engagement (TIMELY) and new Continuing Medical Educatio
Read ArticleLow COVID-19 Vaccine Risks in Rheumatic Patients
Data from the European Alliance of Associations for Rheumatology Coronavirus Vaccine registry shows that patients with with inflammatory or noninflammatory rheumatic and musculoskeletal disease have similar adverse event rates as the general public.
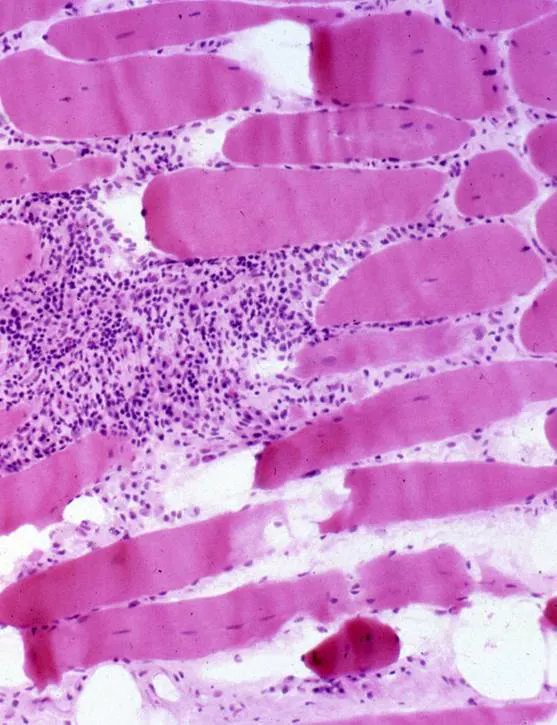
Read ArticlePredicting Treatment Response in Inflammatory Muscle Disorders
Rheumatologists looking for clues to whether their patients with certain inflammatory myopathies will improve on immunosuppressive drugs may find them in a new study appearing in Arthritis Care & Research.
Read Article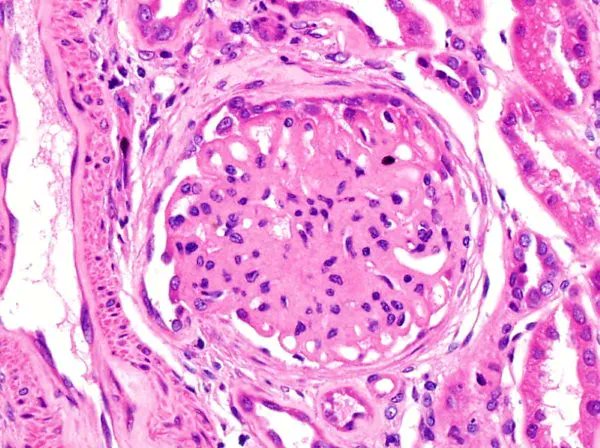
Links:

Links:
Links:

Links:

Links:

Links:
Dr. John Cush RheumNow ( View Tweet)

Links:
Dr. John Cush RheumNow ( View Tweet)

Links:
Dr. John Cush RheumNow ( View Tweet)

Links:
Dr. John Cush RheumNow ( View Tweet)

Links:
Dr. John Cush RheumNow ( View Tweet)
Links:
Links:

Links:
Dr. John Cush RheumNow ( View Tweet)