All News

The evidence ... or lack of .... is there...we cannot recommend the use of medical cannabis to our patients with #RMDs... it may provide some pain relief.. but can cause issues with sleep, fatigue ... and high risk of harm... so early days...! #EULAR2019 https://t.co/CHV5PKexx8
BRITSpA britspauk ( View Tweet)

#Cannabis for the joints? Some conclusions from Prof Serge Perrot at #EULAR2019: No valid clinical trials of medical cannabis in #rheumatology patients. Insufficient evidence about the benefit of pharmaceutical cannabinoids in fibro, OA, RA. Evidence of a high risk of harm. https://t.co/rNzfxo6HyY
Arthritis Ireland Arthritisie ( View Tweet)

Nice SLR done by Gabriele De Marco @LeedsTeamSpA @LeedsBRC @LeedsHospitals in collaboration with GRAPPA in early #PsoriaticDisease ... significant lack of data for treatment sets research agenda! #EULAR2019 https://t.co/MTmGD4Gge3
Leeds Spondyloarthritis Team LeedsTeamSpA ( View Tweet)
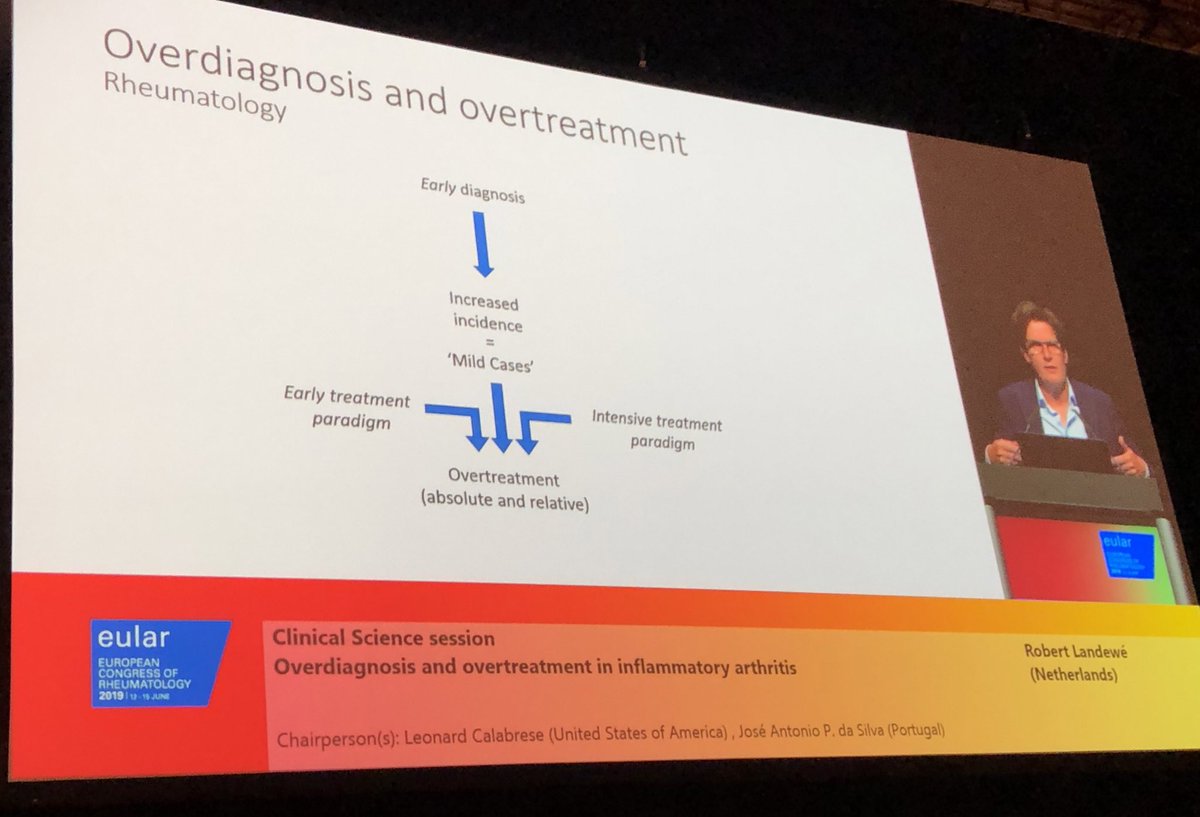
Landewe: reminding us of risks of overdiagnosis & overtreatment.
“It appears that the boundary between feeling ill and being ill has become blurred”
“Pharma is searching for diseases that don’t exist yet”
#EULAR2019 @RheumNow https://t.co/006q0svQpR
Stefan Siebert StefanSiebert1 ( View Tweet)

What gives a false positive ANCA? FRI649 #EULAR2019 @RheumNow
Janet Pope Janetbirdope ( View Tweet)

Pregnancy in rheumatic disease🤰🏻
👉🏻Pre-pregnancy counselling to explain risks & stratify
👉🏻Outcome dependent on disease activity & systemic involvement
👉🏻High-risk pregnancies need multidisciplinary care
👉🏻Many drugs have an acceptable benefit-risk profile in pregnancy
#EULAR2019 https://t.co/guxXsgQoJE
Juan Ovalles, MD, PhD DrJuanOvalles ( View Tweet)

RheumNow expanded twitter coverage of the #EULAR2019 annual meeting is sponsored by Horizon Pharma. All content chosen by RheumNow & its Faculty.
Dr. John Cush RheumNow ( View Tweet)

What do you use first line in fibromyalgia? SP0120 #EULAR2019 @RheumNow
Janet Pope Janetbirdope ( View Tweet)
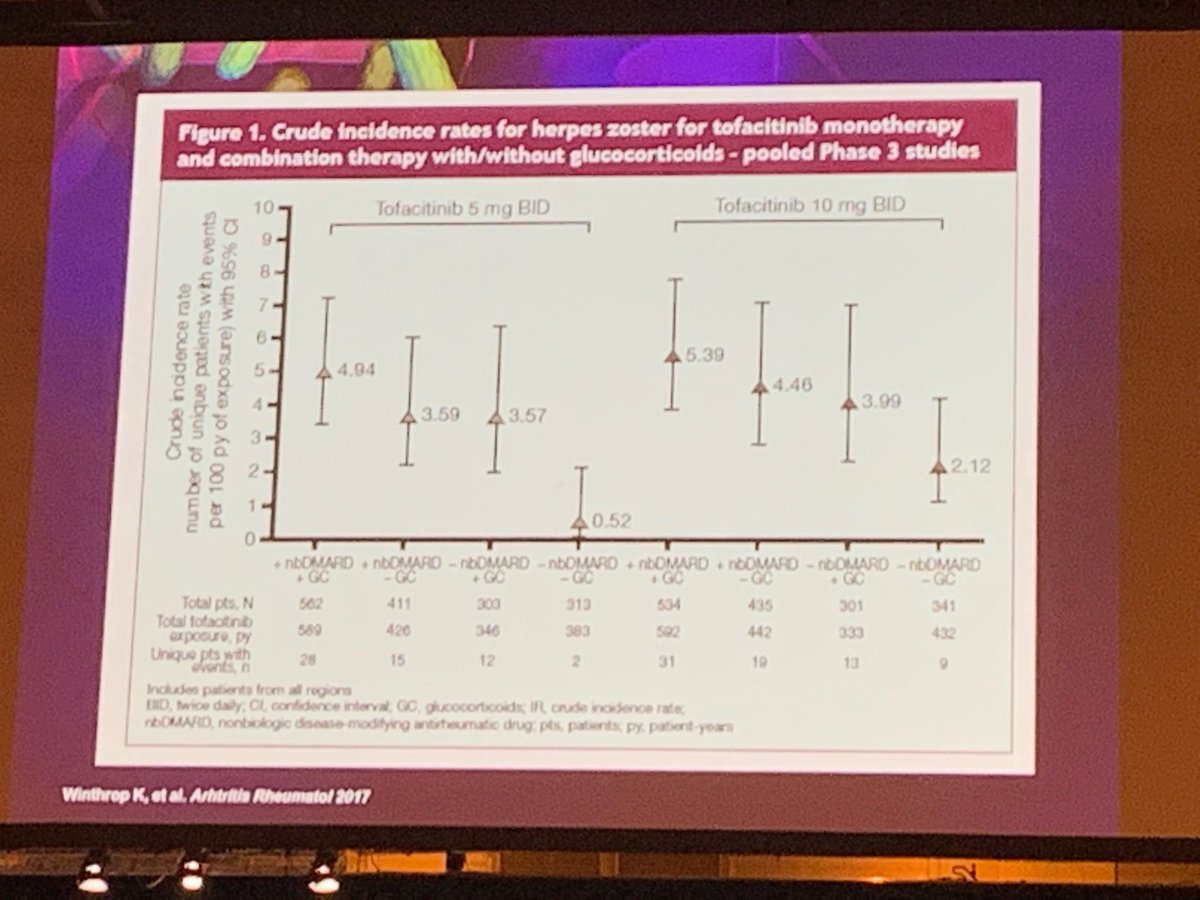
If you can take away concomitant steroid use from Tofacitinib, can reduce zoster risk #EULAR2019 https://t.co/lifzLzqyOt
Dr Irwin Lim _connectedcare ( View Tweet)
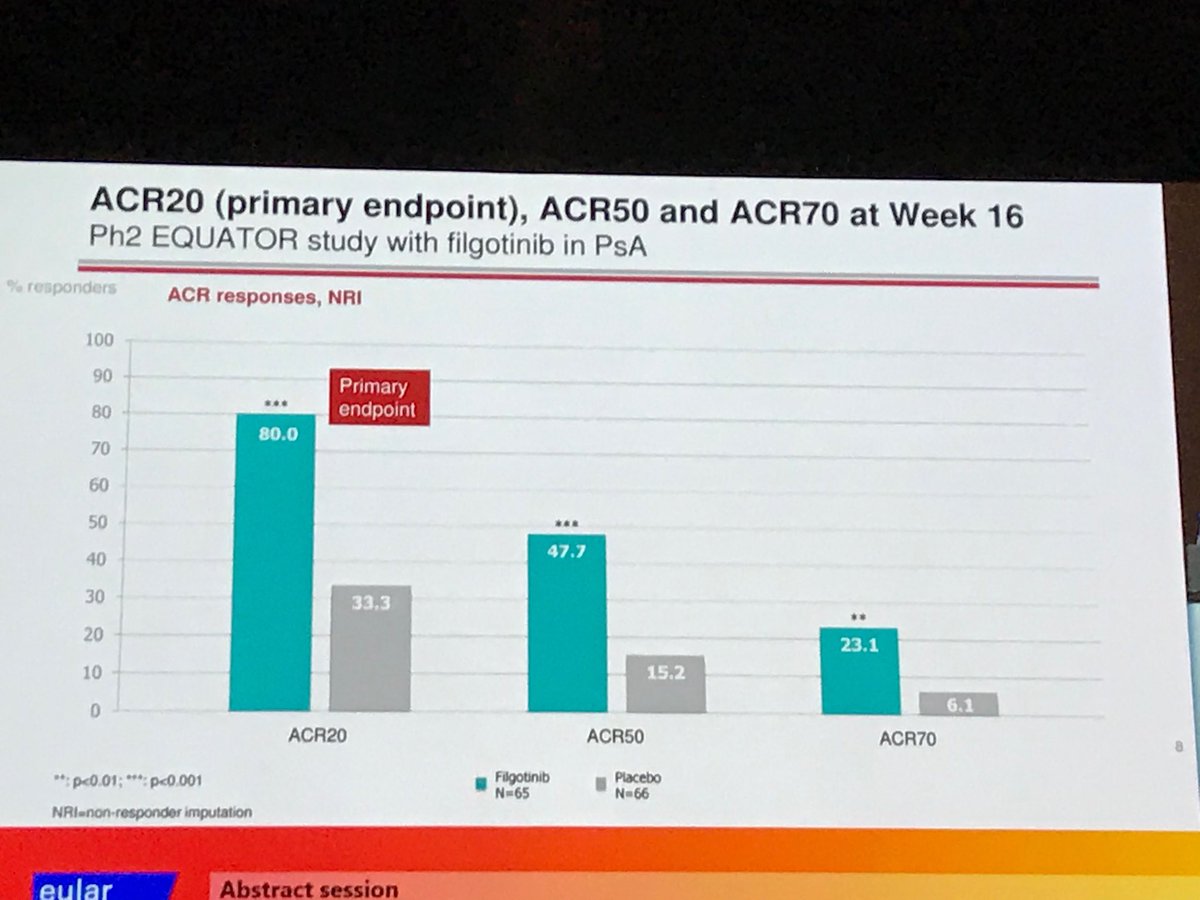
#EULAR2019. Remarkable ACR20 of 80% with Filgotinib in phase II study in PsA. https://t.co/TU5bpixE2y
Patrick Kiely pkiely500 ( View Tweet)
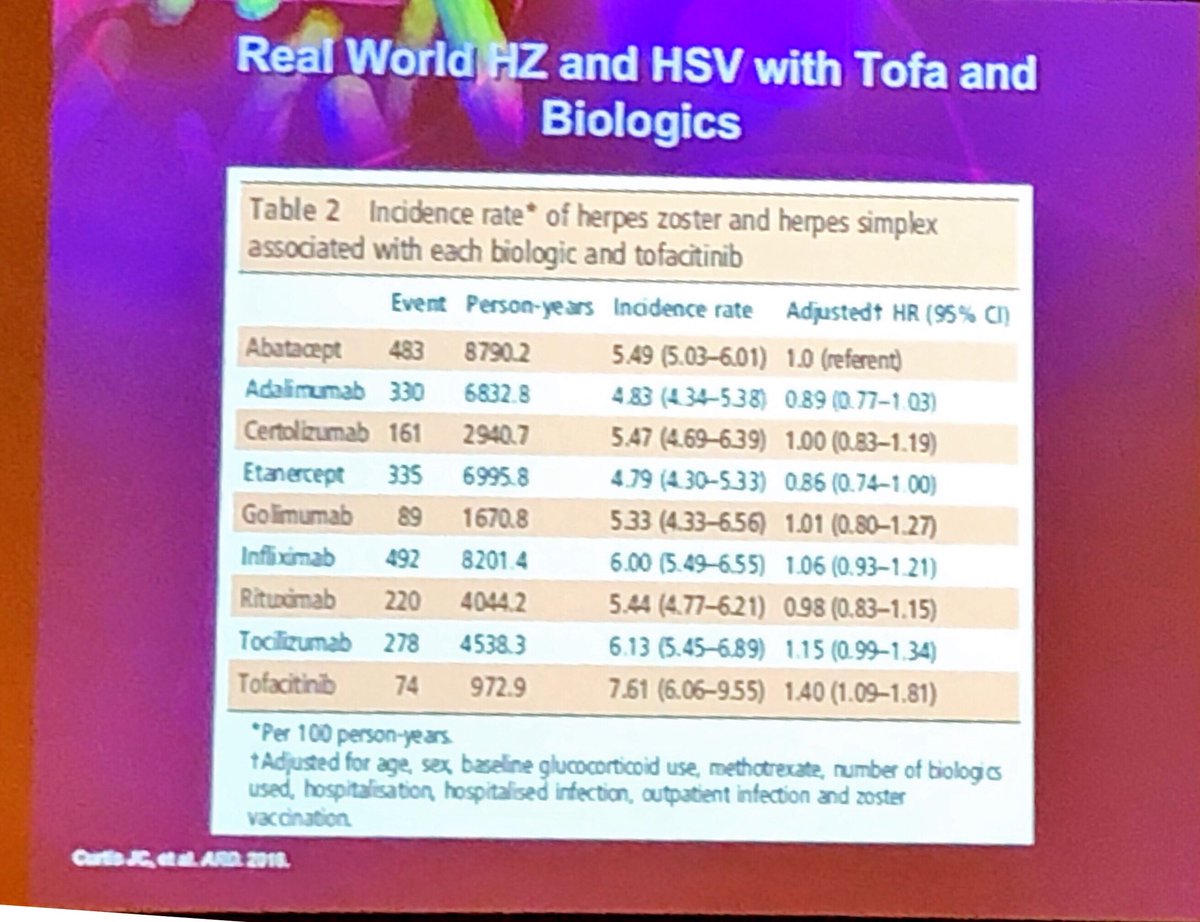
Herpes and biologics and tofa #EULAR2019 https://t.co/UBr0WrV70L
Sylvain La Batide sylba2308 ( View Tweet)
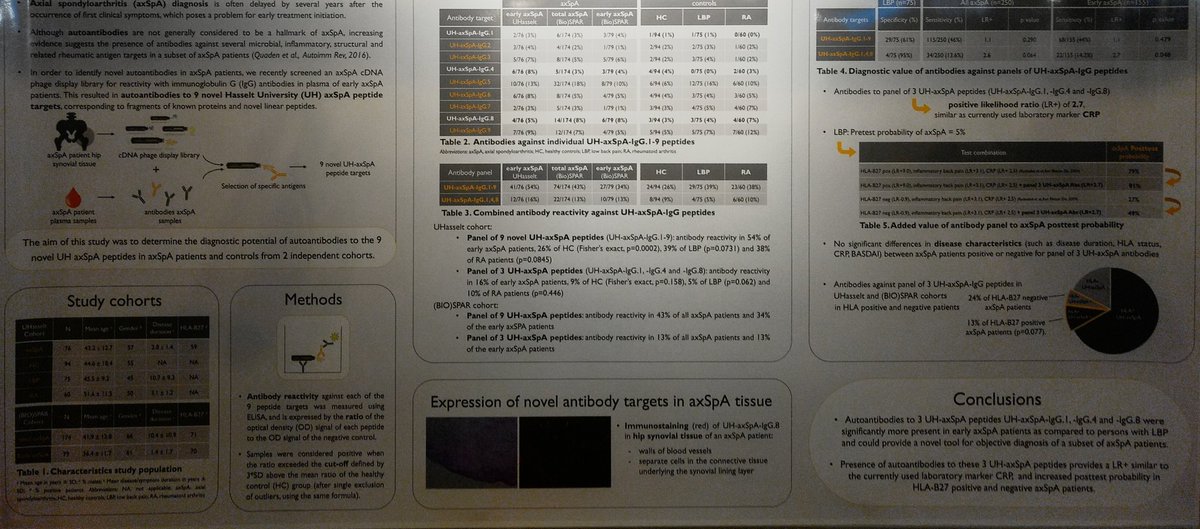
#EULAR2019 Great work looking for potential biomarkers in axial #spondyloarthritis and specifically looking for tissue antigens in the sacroiliac joint. Really this is an unmet need In the filed https://t.co/k9XHUPquRr
WilsonBautistaM WilsonBautistaM ( View Tweet)

Actually the paper says to hold MTX for 2 weeks after flu vaccine. No dose adjustments needed https://t.co/BYE0PKDoPl
Dr. John Cush RheumNow ( View Tweet)

Trial of LIVE zoster vaccine in active TNFi users -> No cases of vaccine HZ!
#EULAR2019 https://t.co/tkYS5VC1yk
Dr Irwin Lim _connectedcare ( View Tweet)
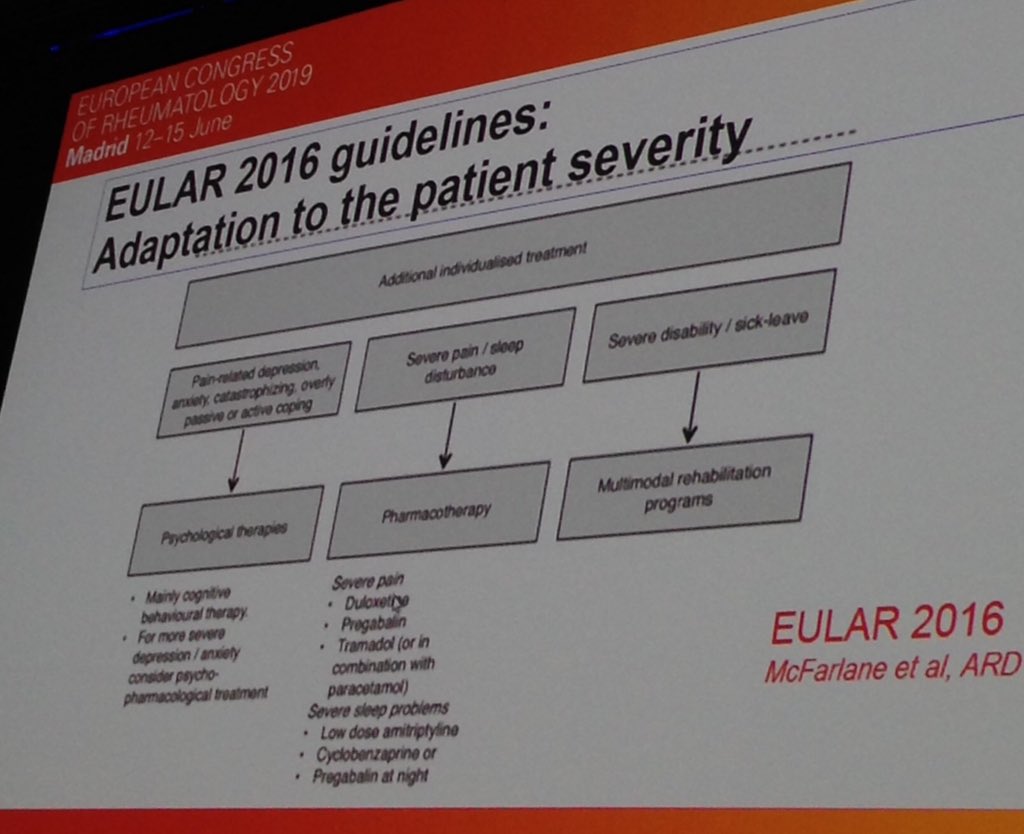
New concepts in FM (fibromyalgia). Diff types of pain likely warrant diff Rx. FM is nociplastic so cranial stimulation , cognitive behavioural therapy etc. Maybe that is why current Rx has low benefits such as meds S#EULAR2019 @RheumNow SP0120 https://t.co/MkqJinQe3p
Janet Pope Janetbirdope ( View Tweet)

In Jak we trust? #EULAR2019 https://t.co/pD0wWXtDNw
mrreutt matylda68 ( View Tweet)
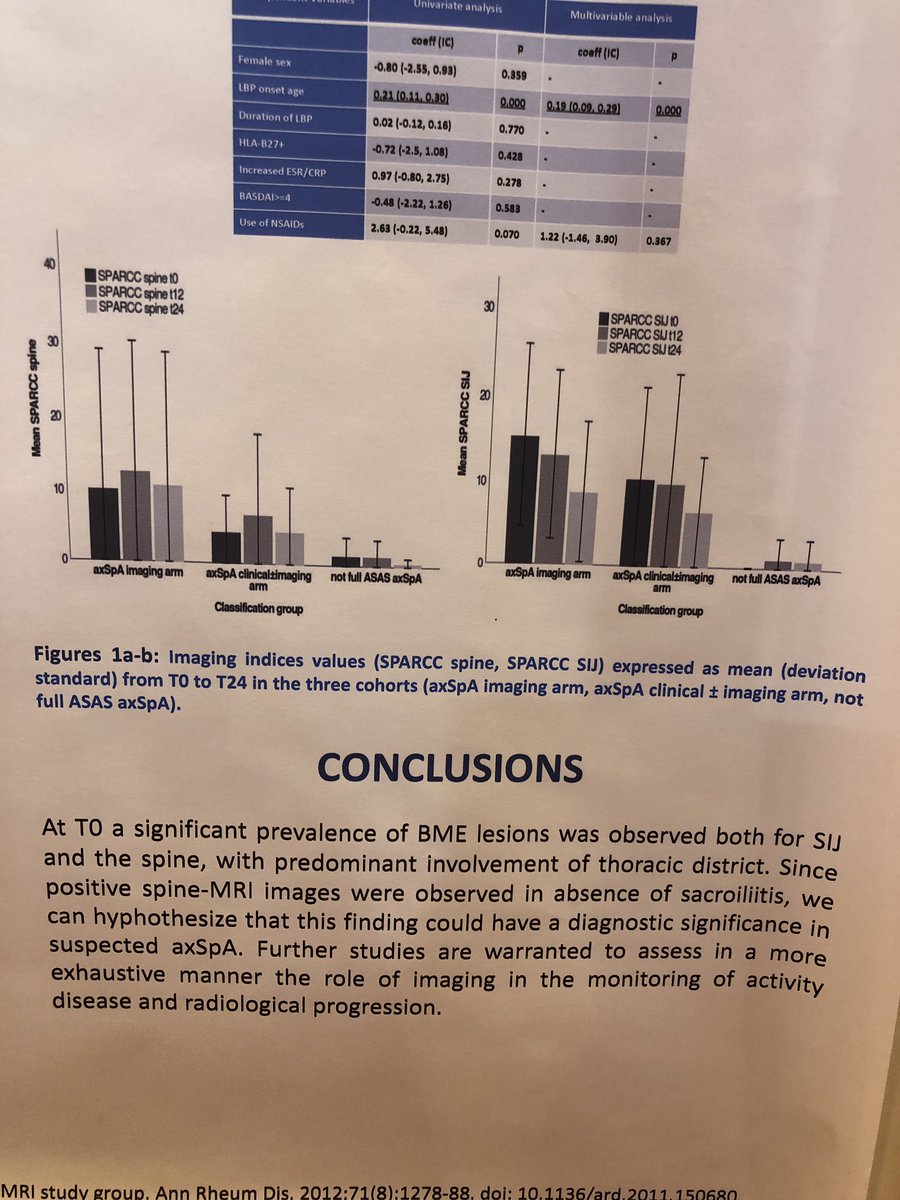
Interesting findings from an AxSpa study at the #eular2019 #rheumatology conference: spine MRIs may be used to diagnose lesions in the spine before evidence of sacroiliitis, which has diagnostic implications for early AxSpa @CreakyJoints https://t.co/rez9G7iExI
Kelly Gavigan KellyLGav ( View Tweet)
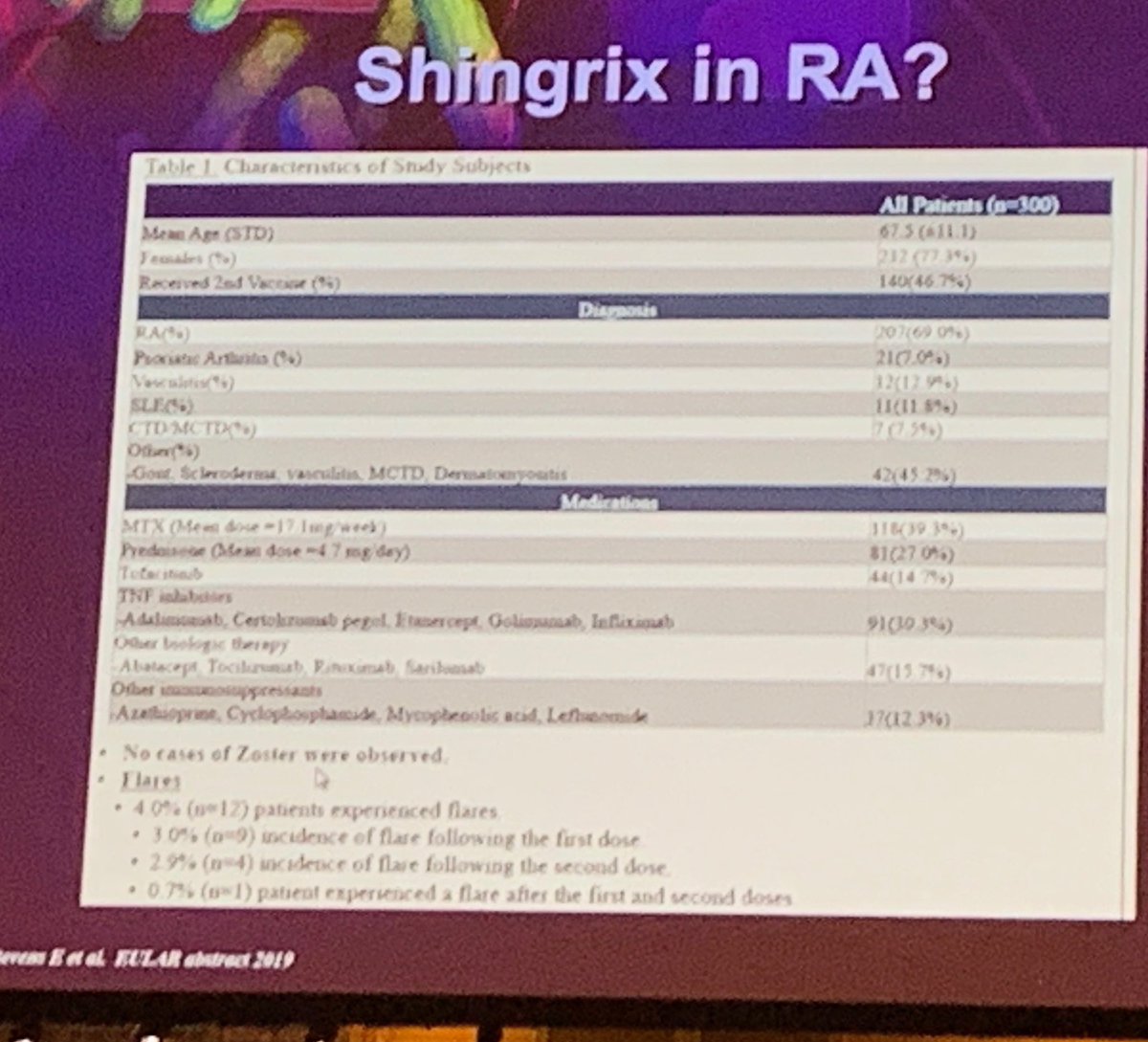
Shingrix in RA. Due to the potent immunogenicity of the adjuvant, there’s some concern it might flare RA. Study reported #EULAR2019 not showing flare. https://t.co/nrHRYdP6eN
Dr Irwin Lim _connectedcare ( View Tweet)
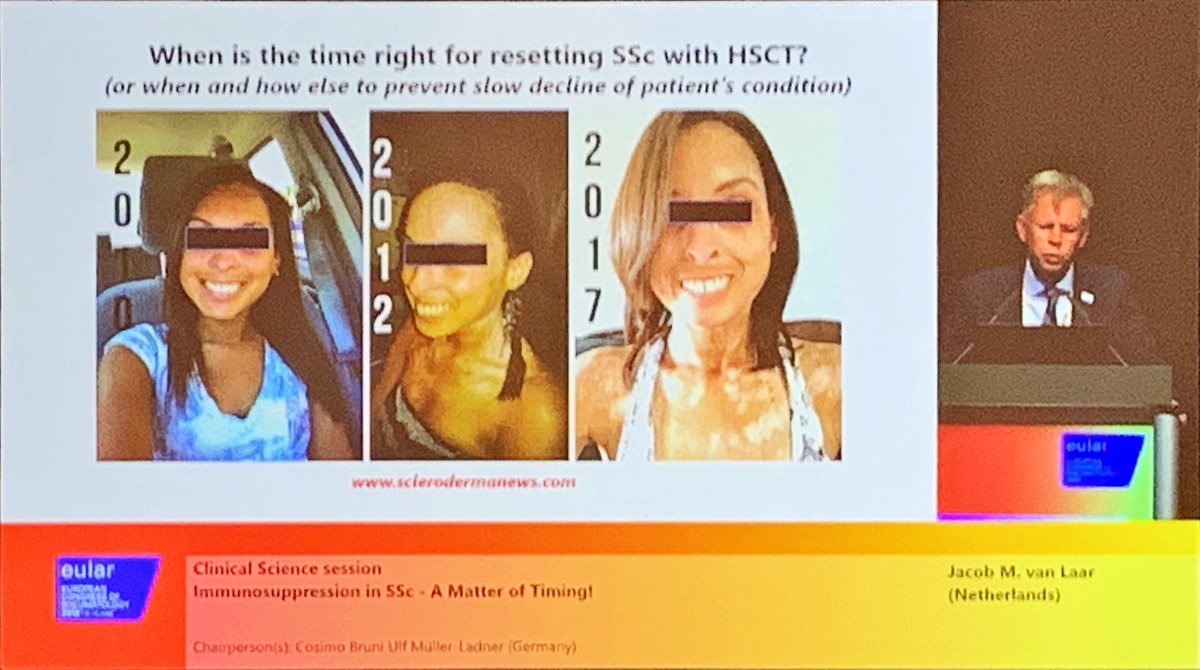
How do we pick which scleroderma patients are stem cell transplant candidates early enough? “Patient selection is the critical thing in the whole transplant venture” - our patients have damage and aren’t as fit. Jacob van Laar at #EULAR2019 SP0113 @RheumNow https://t.co/cDuAI7iVWk
David Liew drdavidliew ( View Tweet)
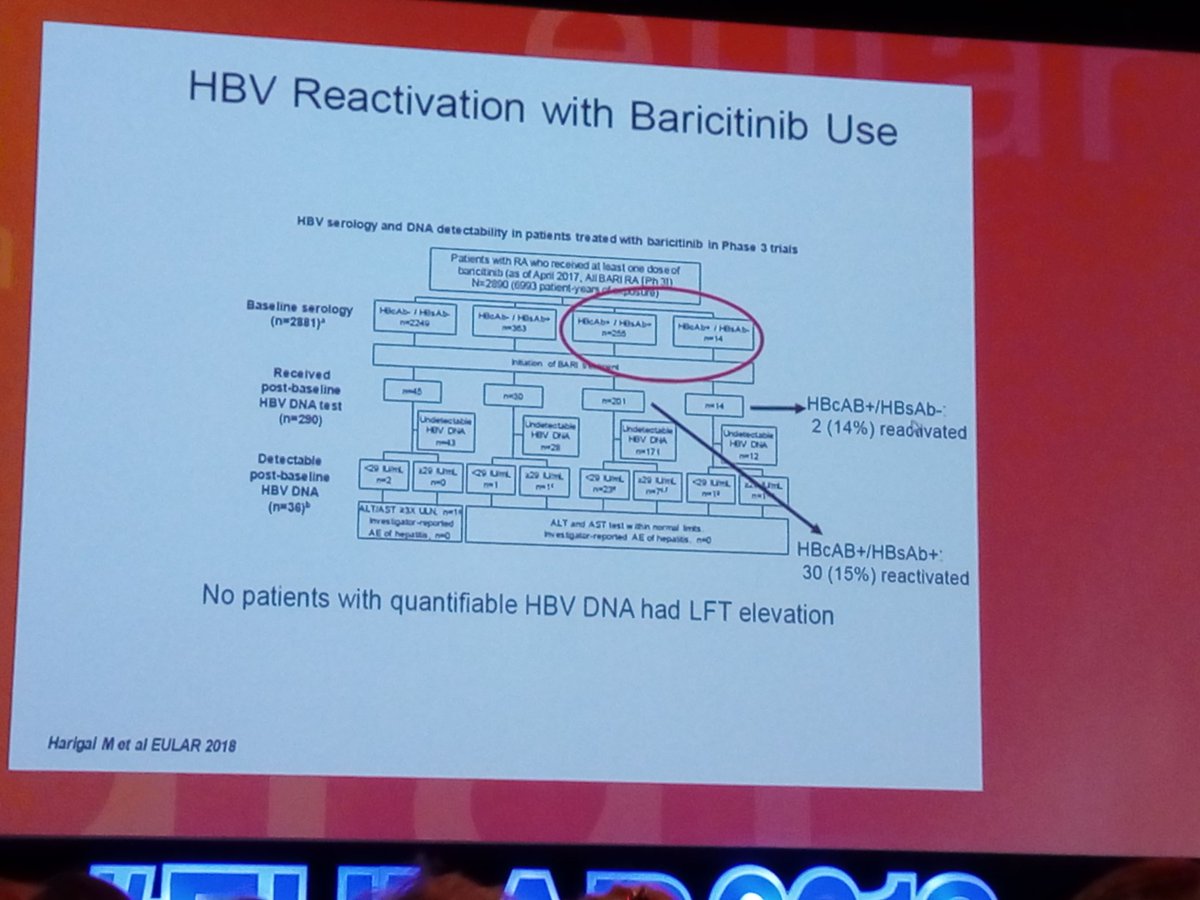
HBV reactivation with #Baricitinib use. The JAKi may causes reactivation of virus - Kevin Winthrop @OHSUNews #EULAR2019 https://t.co/phV0hVUH2Y
Dr.Martínez MtzReuma ( View Tweet)


