All News
Hematologic Malignancies Increased in Systemic Sclerosis
Swedish national study found that among a cohort of systemic sclerosis (SSc) patients a two-fold increase risk of hematological malignancies was seen.
Read ArticleLong-Term APP Retention: Building Success for Practices and Providers
Advanced Practice Providers (APPs) play an increasingly vital role in rheumatology practices across the country. With rising patient volumes and a growing demand for timely, high-quality care, incorporating an APP into a rheumatology team can be transformative.
Read ArticleTurkey Tryptophan (11.28.2025)
Dr. Jack Cush reviews the news and reports from this past week on RheumNow.com, including reports on FDA resurrections, FM seasonal worsening, and do you fight switch or swap biologics in PsA TNFi nonresponders?
Read Article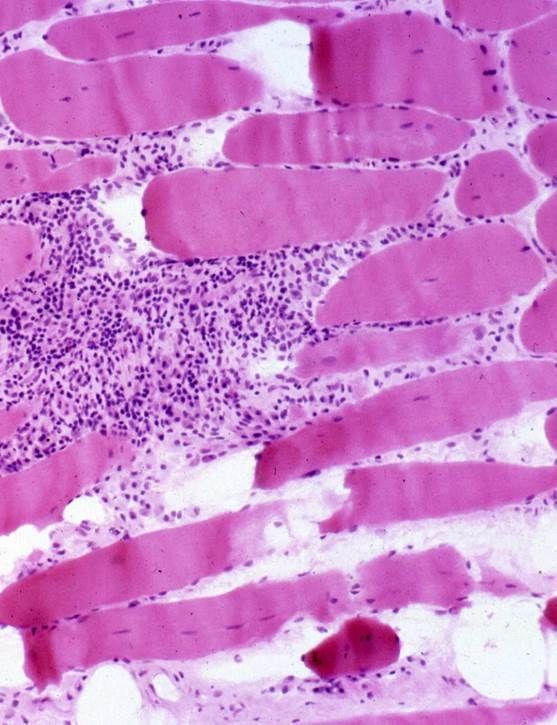
Scandinavian study of > 13.6 million PYs shows NO increased risk of myositis after SARS-CoV-2 (mRNA & adenoviral vector) vaccines. Data from 101 myositis events in 7,002 398 unvaccinated PYs. Adj IRR 180d risk was 0.84 (0.63–1.11) and 1.31 (0.72–2.36) respectively https://t.co/D5KVyvXaot
Dr. John Cush RheumNow ( View Tweet)

RNL 25 Replay: Vasculitis
Enjoy these excerpts from the RNL 2025 meeting, and join us for RNL 2026 in Dallas, TX - registration is now open! Visit https://t.co/o78Pc8LPsQ for complete details. Featured in this video are excerpts from the following: - Dr. Michael Wechsler: https://t.co/Rk7sBosL72
Links:
Dr. John Cush RheumNow ( View Tweet)
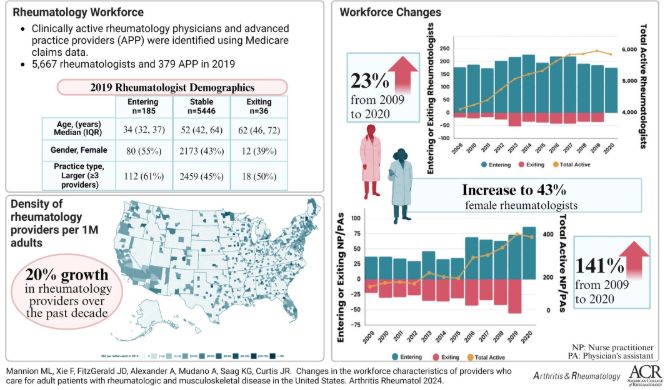
A&R USA adult rheum workforce study showed in 2019, there were 5,667 rheumatologists & 379 APPs. Betw 2009 - 2020, rheumatologist #s increased 23%, while #APP #s increased 141%. There was a signif increase in female rheumatologists & APPs over time https://t.co/Y5ZSwPLsNe https://t.co/RzDxbN7irL
Dr. John Cush RheumNow ( View Tweet)
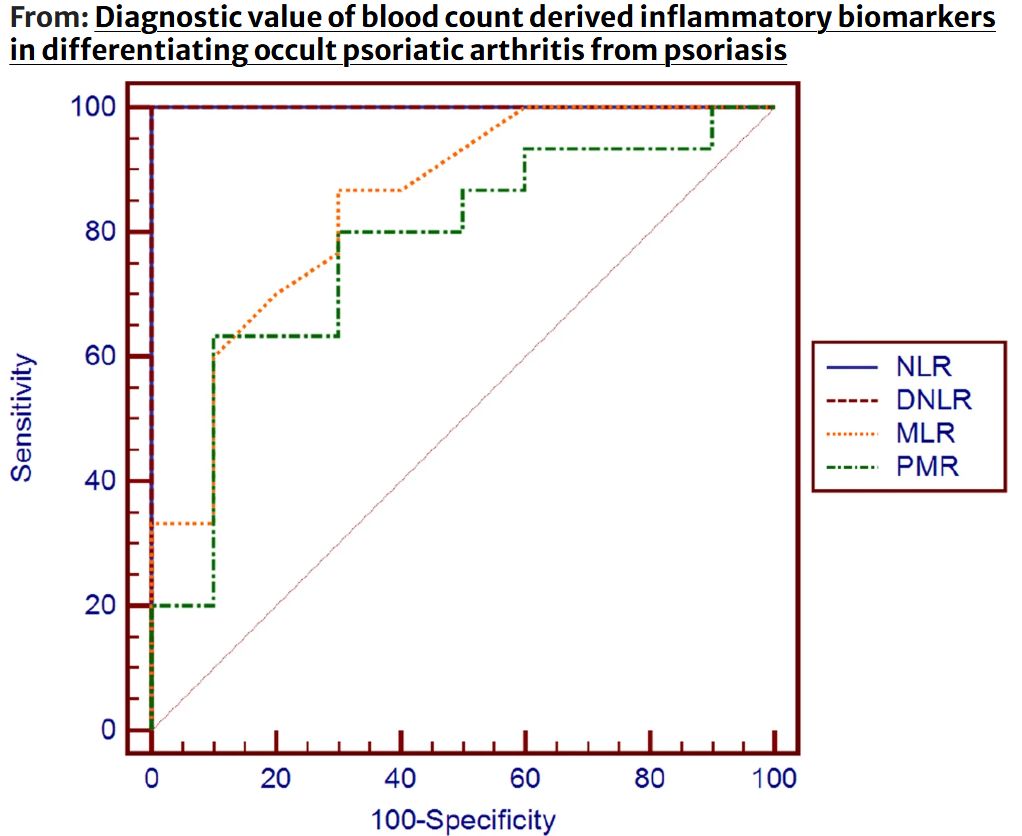
CBC & Psoriasis - case–control study 60 pts: 30 PsA & 30 PSO pts showed that PsA showed elevated WBCs, neutrophils, monocytes & inflammatory markers (NLR, MLR, SII, SIRI, & decr PMR (all p < 0.001). NLR 100% Dx accuracy (AUC = 1.000). WBC > 6.7, MLR > 0.17, & PMR ≤ 503 were https://t.co/AB7iFiMuSM
Dr. John Cush RheumNow ( View Tweet)

Congratulations to Professor Stuart Ralston (Versus Arthritis Professor of Rheumatology) from the University of Edinburgh. He has been honored with the RSE Sir James Black Medal by The Royal Society of Edinburgh for his research research & dedication to medical education https://t.co/vCm4Mkp6u7
Dr. John Cush RheumNow ( View Tweet)
Taiwans insurance claims, Maternal Database, & BirthRegistry examined 42,493 births to fathers w/ autoimmune Dz, 14.3% exposed to immunosuppressants or biologics. MTX posed no risk, but Cyclosporin, AZA, JAKi may be assoc w/ adverse preg outcomes, but event #s small. https://t.co/6JXaZ2HdAF
Dr. John Cush RheumNow ( View Tweet)

Treating Refractory Still's Disease
A full read systematic review article examined treatment options for difficult to manage systemic juvenile idiopathic arthritis, or Still’s disease. It classified these refractory disease patients as those with either (1) persistent https://t.co/uDeIkIek1d
Dr. John Cush RheumNow ( View Tweet)

DERM on RheumNow (November 2025)
The Derm on RheumNow podcast is a collection of Citations and Content curated for dermatologists – addressing Psoriasis, PsA, CLE, vasculitis, HS, other CTD skin disorders. dermatology drugs, biiologics, JAKs - their use, efficacy and side https://t.co/mX0Hv1RRF9
Dr. John Cush RheumNow ( View Tweet)

RheumNow Live 2026 is coming February 7th and 8th to Dallas, Texas - and registration is now open!
Ready for the most interactive, engaging and practice-changing rheumatology meeting of the year?
This year’s program covers vasculitis, autoimmune disease, PsA, RA outcomes, https://t.co/cftvdSn3t7
Dr. John Cush RheumNow ( View Tweet)

"When you learn, teach. When you get, give." – Maya Angelou
Dr. John Cush RheumNow ( View Tweet)

BASDAI scores elevated in Fibromyalgia - 58 FM pts were surveyed wtih FIQ, SSS, BASDAI. BASDAI was ≥ 4 points in 91.4% of FM (vs 13% controls (p < 0.001). BASDAI correlated w/ the FIQ (pain, fatigue, stiffness) & SSS scores (r = 0.88, p < 0.00). Could BASDAI be used in FM? https://t.co/M0tI8wjzh4
Dr. John Cush RheumNow ( View Tweet)

Partial vs Total Knee Replacement: Is One Superior?
Patients with osteoarthritis (OA) primarily affecting only one knee joint compartment did just as well with partial knee replacement (PKR) as with total arthroplasty after 10 years in a randomized trial -- and maybe a little https://t.co/KOZwedtnxo
Dr. John Cush RheumNow ( View Tweet)

Zantac is Back! Ranitidine has been off the market for 5yrs, withdrawn over concerns about N-nitrosodimethylamine (NDMA) impurity affecting shelf-life. The FDA has approved reformulated ranitidine tablets - the drug has the same prior efficacy and safety label. https://t.co/ktTz5EO2Ja
Dr. John Cush RheumNow ( View Tweet)
Retrospective study of 39 pts w/ MDA5 + DM-ILD Rx w baricitinib. 31 (79.5%) had improvement in Gottron’s, heliotrope, dyspnea, HRCT score, ferritin, LDH, steroid dose & 6 mo survival (87% vs. 70%, p = 0.047). https://t.co/RCTbBsCkeV https://t.co/dF8I0gF4Dy
Dr. John Cush RheumNow ( View Tweet)
A New Disease Activity Score for Antiphospholipid Syndrome?
APS is a systemic autoimmune disorder. It is characterised by the presence of antiphospholipid antibodies and a broad spectrum of clinical features, causing obstetric as well as thrombotic, microvascular and https://t.co/G2xGtOPmwb
Dr. John Cush RheumNow ( View Tweet)

RheumNow Live 2026 is coming February 7th and 8th to Dallas, Texas - and registration is now open!
This year’s program covers vasculitis, autoimmune disease, PsA, RA outcomes, spondyloarthritis, and much more.
Reserve your spot today at https://t.co/o78Pc8LPsQ https://t.co/GOUW5Od9Hy
Links:
Dr. John Cush RheumNow ( View Tweet)
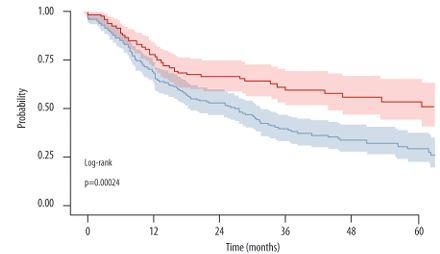
Study of 452 #PsA pts who failed 1st line TNFi Rx (LOE) - 275 Cycled to another TNFi vs 177 Swapped to an IL-17i. Higher Retention in Swap (78%) vs cycled (68%) PsA pts (p < 0.001) @12 mos (60 v 40%@3yrs). Tx failure predicted by cycling, Dz Activity, Rx year, axial & mixed https://t.co/VQLBlBEw4W
Dr. John Cush RheumNow ( View Tweet)






