All News
Review: Monoclonal Gammopathy of Undetermined Significance
NEJM has reviewed the common hematologic/immunologic finding of "monoclonal gammopathy of undetermined significance" (MGUS) - a common premalignant plasma-cell proliferative disorder found in approximately 5% of those over age of 50 years.
Read ArticleGender Complexities in Psoriatic Arthritis Treatment Outcomes
Analysis of a German psoriatic arthritis (PsA) patients registry demonstrates sex-specific differences regarding clinical manifestation and treatment outcomes, especially with regard to drug discontinuations.
Read ArticleOptimizing DMARD Therapy in Rheumatoid Arthritis
A phase IV trial from Spain demonstrates that optimization of therapy by lessening biological DMARDs in rheumatoid arthritis (RA) may be feasible, with a low risk of flare.
Read ArticleNot all difficult-to-treat RA will have rapid radiographic progression
A study of radiographic outcomes in difficult-to-treat rheumatoid arthritis (D2T RA) and poly-refractory RA (pr-RA) show a subset in whom damage is rapid and in need of more aggressive therapy.
Sequencing DNA to find new lupus treatments
Medical University of South Carolina geneticist Betty Tsao, Ph.D., will lead a five-year project to identify rare mutations associated with childhood-onset systemic lupus erythematosus (SLE), or lupus, with more than $3.5 million in funding from the National Institute of Arthritis and Musculoskeletal and Skin Diseases (NIAMS). Tsao holds the Richard M. Silver Endowed Chair for Inflammation Research in the Division of Rheumatology and Immunology at MUSC.
Read ArticleAssociations in Rheumatology (10.3.2025)
Dr. Jack Cush reviews the news, journal reports and important associations in rheumatology from the past week on RheumNow.com.
Read ArticleGuselkumab FDA Approved for Pediatric Psoriasis and Psoriatic Arthritis
FDA announced yesterday that guselkumab (Tremfya) is approved for use in pediatric patients moderate to severe plaque psoriasis (PsO) or active psoriatic arthritis (PsA) in children six years and older (weighing at least 40 kg).
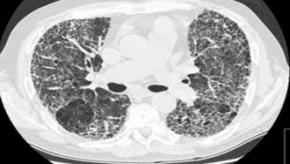
Read ArticleHigh-Resolution CT in CTD-Interstitial Lung Disease
A systematic review and meta-analysis examined the role of high-resolution computed tomography (HRCT) in diagnosing and characterizing interstitial lung disease (ILD) associated with connective tissue diseases (CTDs).