All News
Ten-Year Analysis of Low-Dose Glucocorticoids in early RA
Abstract #1998 by C. Roubille et al from Montpellier, France is a 10-year prospective analysis that looked at the risk of severe outcomes related to very low-dose glucocorticoid in early rheumatoid arthritis from the ESPOIR cohort.
Read ArticleRheumatology Round-Up with Drs. Kavanaugh & Cush
The Annual Review of Best ACR 2020 abstracts featuring Drs. Cush and Kavanaugh filmed on the last day of Virtual ACR 2020 Nov. 9, 2020. Watch now!
Read Article
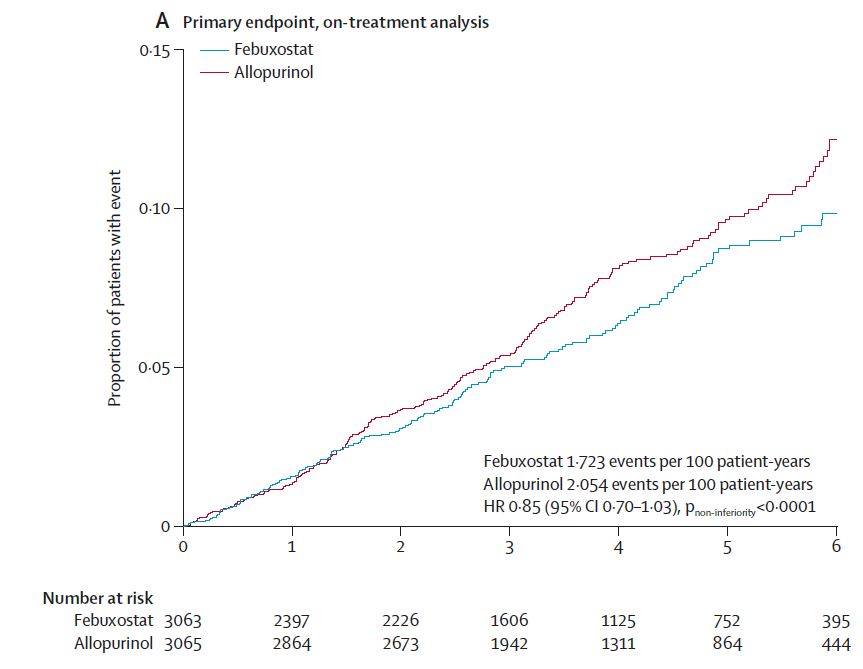
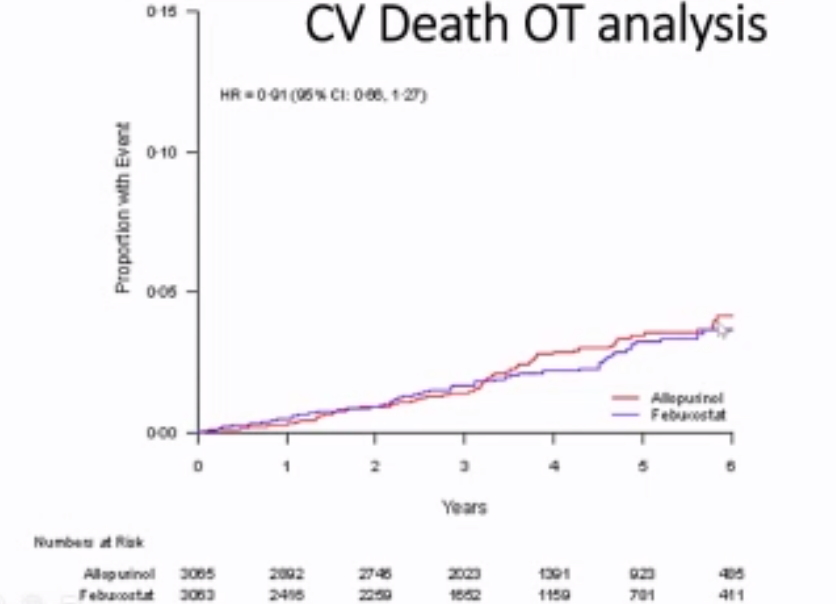
FAST, FREED, CARES, & CONFIRMS: A Run-Down on the Black-Box Blues of Febuxostat
Febuxostat, a nonpurine xanthine oxidase inhibitor used in the treatment of chronic gout to lower serum urate levels, received bad press in 2019 after the US Food and Drug Administration (FDA) placed a black-box warning on the medication’s label suggesting that it increases the risk of cardiovasc
Read ArticleAnswering questions on how COVID-19 affects rheumatic patients
As we inch closer to a possible vaccine for COVID-19, we are finally filling in the details on how the pandemic is affecting every aspect of our patient’s lives.
Read Article
Links:

Links:

Links:
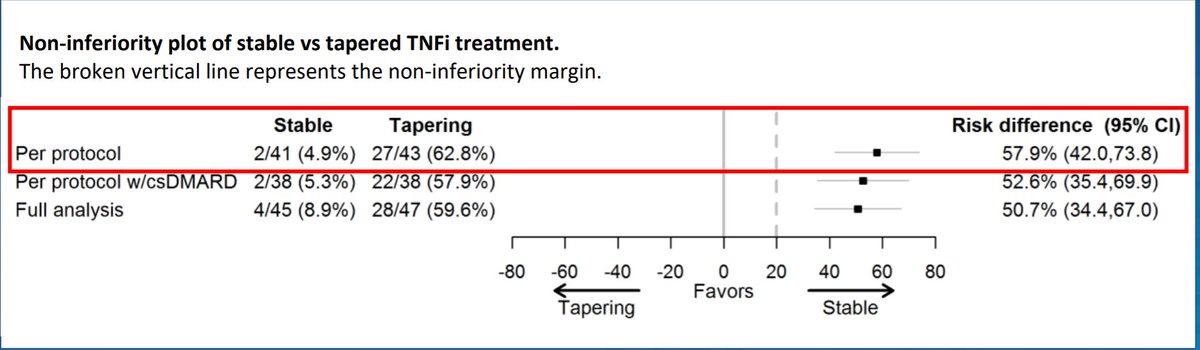
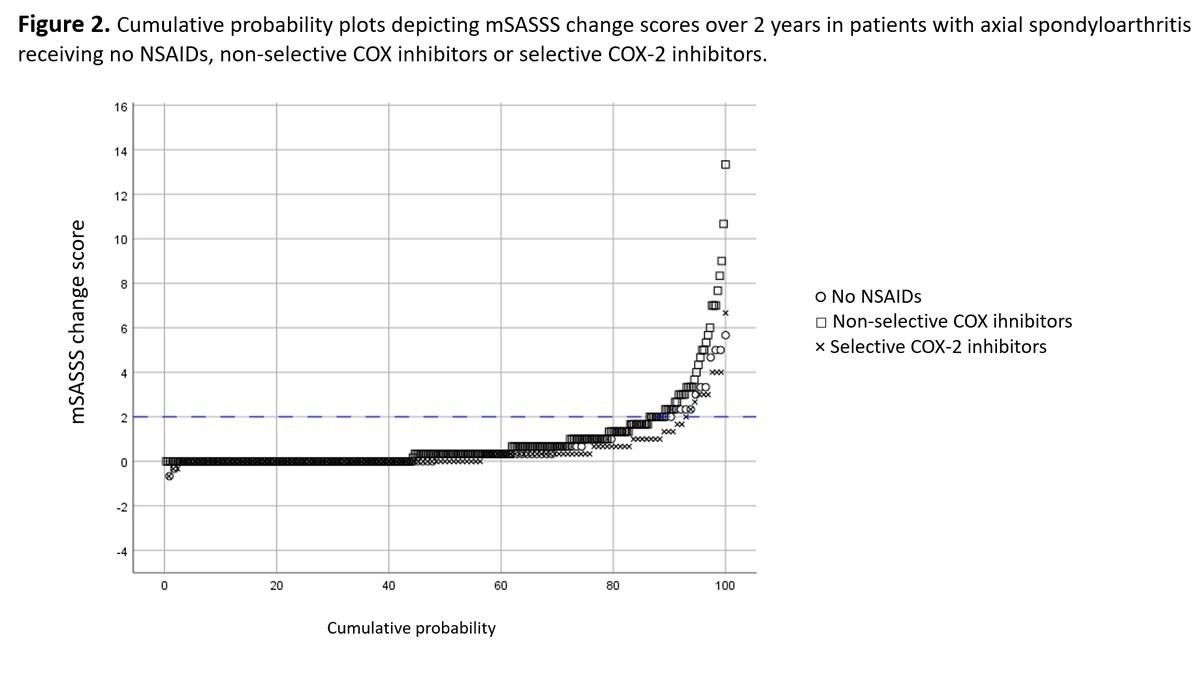

k dao KDAO2011 ( View Tweet)

Dr. John Cush RheumNow ( View Tweet)

David Liew drdavidliew ( View Tweet)

Olga Petryna DrPetryna ( View Tweet)
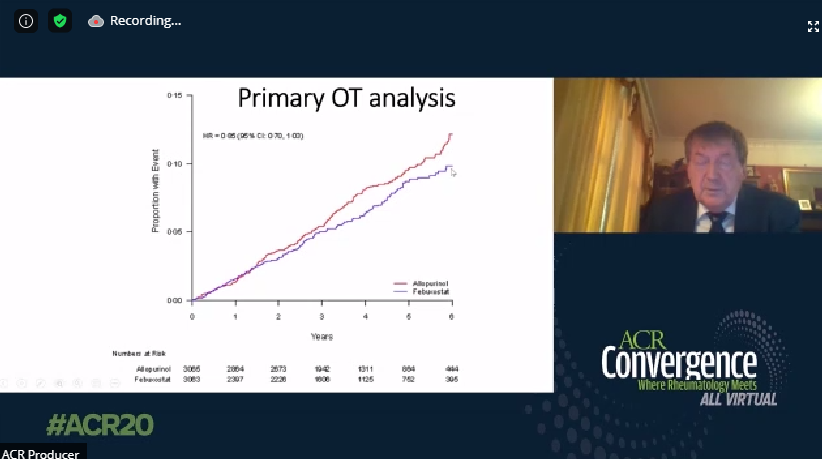

Links:
NatRevRheumatol NatRevRheumatol ( View Tweet)

Richard Conway RichardPAConway ( View Tweet)
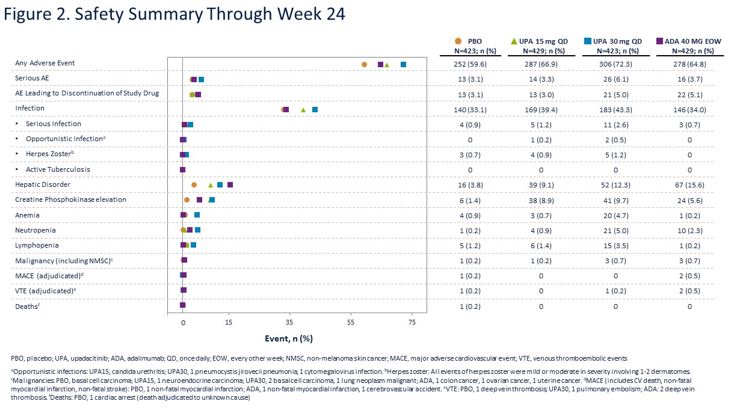
Upadacitinib vs. Adalimumab vs. PBO in PsA Phase 3 RCT ⬇️MSK symptoms, PsO, function, pain, fatigue, rads progression in UPA *⃣UPA15 non-inferior to ADA for ACR20, UPA30 superior *⃣Similar VTE in all arms @RheumNow #ACR20 Abs#2026 https://t.co/OHzIjXKqAu