All News
ILD and ANCA: What to do?
Several cohort studies conducted in Asia, Europe and the Americas have evaluated the clinical significance of antineutrophil cytoplasmic antibodies (ANCA) when detected in patients with interstitial lung disease (ILD) as well as the types of ILD encountered in patients with microscopic polyangiitis (MPA) and their effect on the prognosis of MPA.
Read ArticleCAR-T Product Shows Early Promise in Lupus
Five patients with systemic lupus erythematosus (SLE) in China have been treated with a potentially groundbreaking form of chimeric antigen receptor (CAR) T-cell therapy, researchers reported, with encouraging results after 3 months.
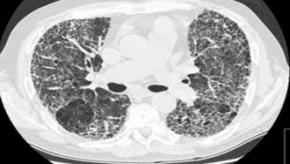
Read ArticleHigh-Resolution CT in CTD-Interstitial Lung Disease
A systematic review and meta-analysis examined the role of high-resolution computed tomography (HRCT) in diagnosing and characterizing interstitial lung disease (ILD) associated with connective tissue diseases (CTDs).
Methylprednisolone plus Methotrexate in Giant Cell Arteritis
What is the optimal glucocorticoids (GC) regimen in giant cell arteritis (GCA)? Does methotrexate (MTX) work in GCA?






Links:


Links: