All News
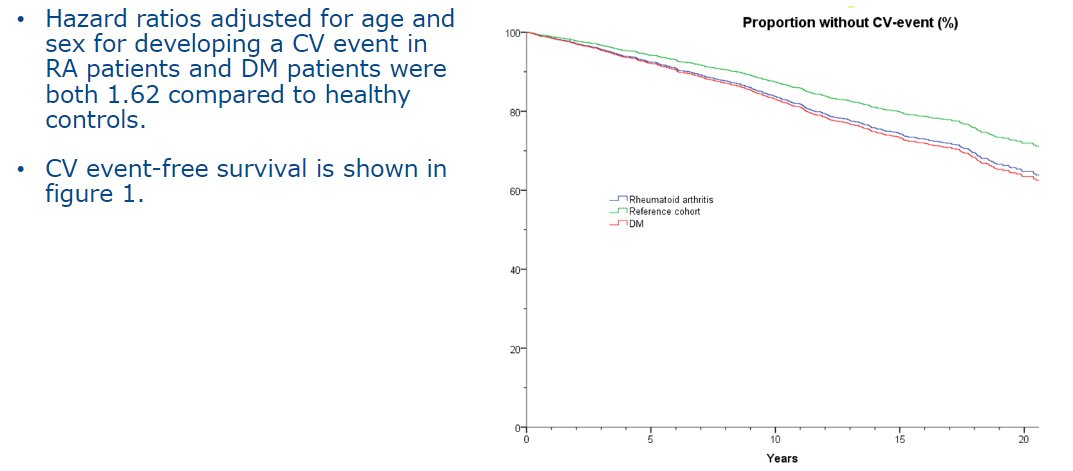
Diabetes puts you at risk of cardiovascular disease, right?
What about RA?
Look at this graph and compare the pair.
"already it was impossible to say which was which” - George Orwell, Animal Farm
#ACR21 ABST0287 @RheumNow https://t.co/r0yaeC530l https://t.co/mKJhoyJ2Cn
David Liew drdavidliew ( View Tweet)

Study by @BethIWallace @Tuhina_Neogi shows that we seem to treat fibromyalgia symptoms with steroids in RA patients. Its a bad idea in fibromyalgia, and has got to be bad practice in RA too. Abstr#0120 #ACR21 @RheumNow
Richard Conway RichardPAConway ( View Tweet)
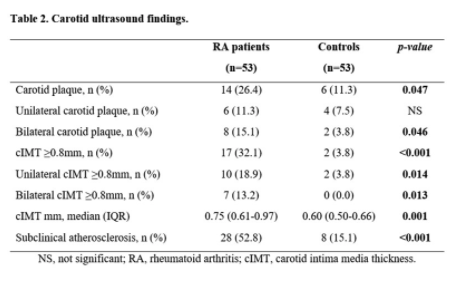
#ACR21 Abst#0271. ⬆️ Risk of carotid atherosclerosis in first few years of RA diagnosis. Authors recommend routine carotid screening in pts at time of diagnosis.
Q: would you routinely screen all patients at time of diagnosis?
@Rheumnow
https://t.co/A9akqyVRGF https://t.co/SYGkJHQzWf
Links:
Eric Dein ericdeinmd ( View Tweet)
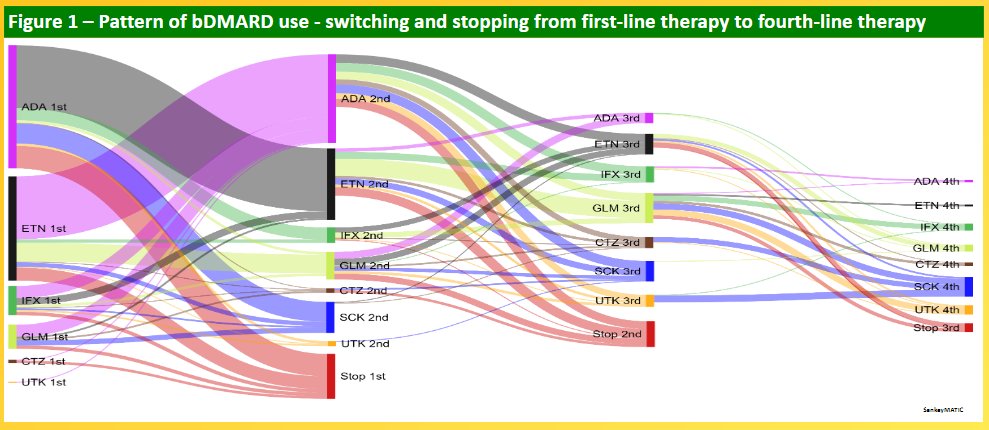
PsA bDMARD use in Australia🇦🇺
(where once you qualify for a bDMARD in general, it's the physician's free choice)
1. cool Sankey diagram showing switches
2. given the chance, people switch in all directions & switch hard
(registry linked with insurance)
#ACR21 ABST0201 @RheumNow https://t.co/jcVPa8jXsR
David Liew drdavidliew ( View Tweet)
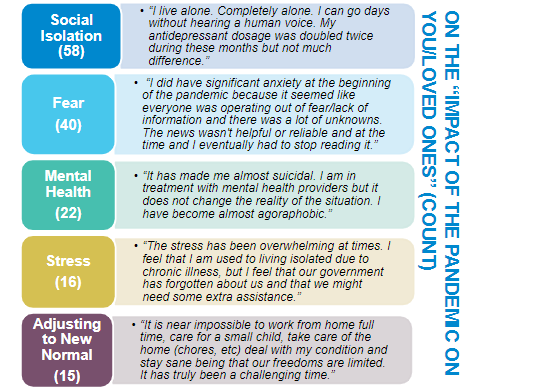
COVID-19 has been tough on everyone, but especially tough on our patients in rheumatology.
We need to think about the long-term impacts, but also about how we can do it better next time.
@HSpecialSurgery #ACR21 ABST0266 @RheumNow https://t.co/JdOqZ8Svin https://t.co/c0rlY9PfDv
David Liew drdavidliew ( View Tweet)
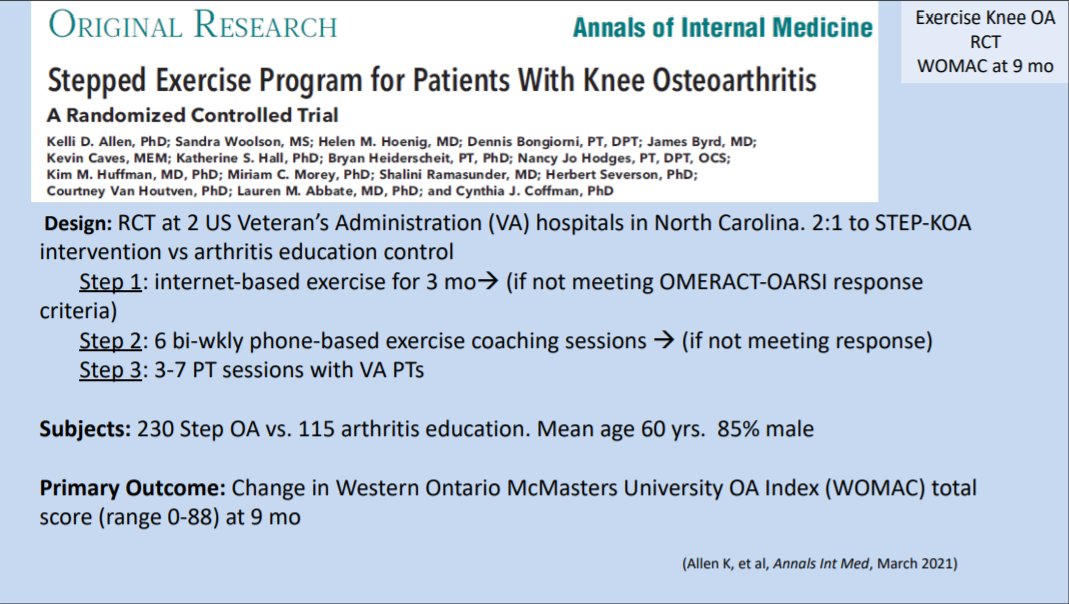
STEP-KOA trial: a promising intervention for knee #osteoarthritis?🤔
@RheumNow #ACR21 #YearinReview https://t.co/6Vkx0WR0tx
sheila RHEUMarampa ( View Tweet)
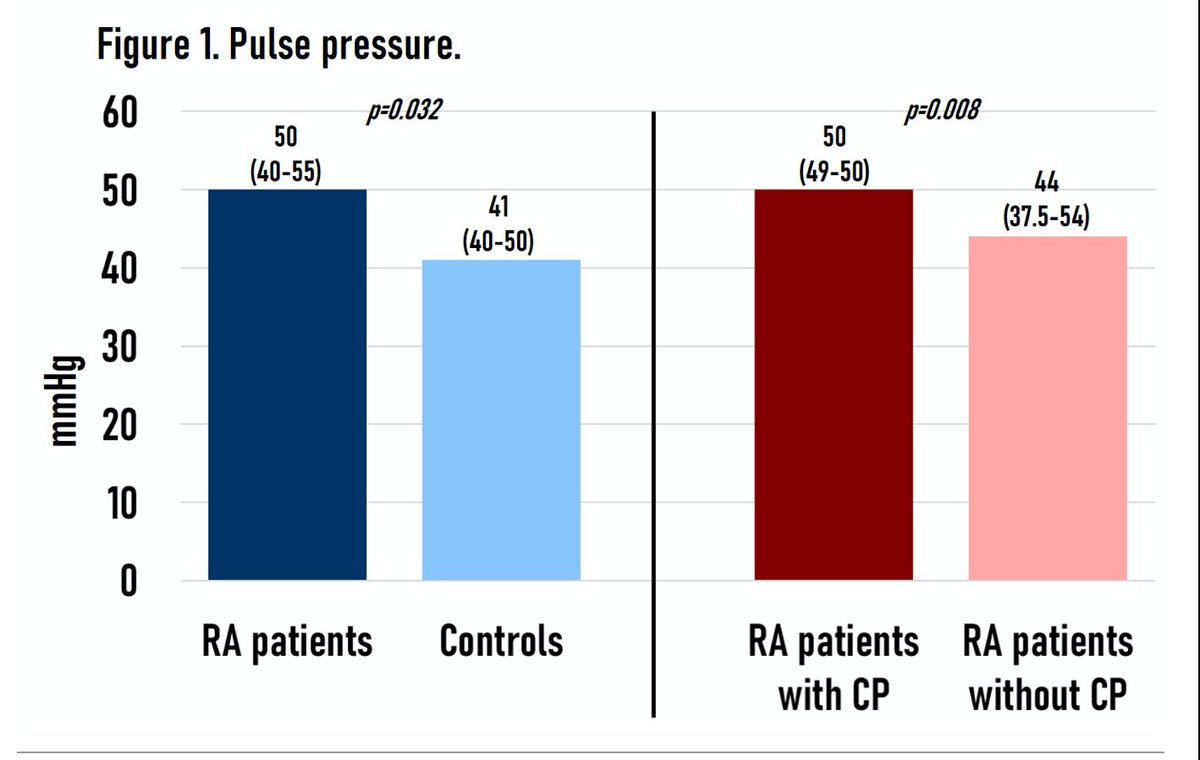
Abst#0276:
Pulse Pressure (PP) can predict carotid plaque in RA!
➡️PP (SBP – DBP) assoc w/ subclinical CVD in gen populat. How about in RA?
⭐︎ RA pts had higher PP than controls
⭐︎ PP = only independent RF for subclinical athero
#ACR21 @Rheumnow https://t.co/esLDW8iggf https://t.co/6d2D9hXC1i
Meral K. El Ramahi, MD MeralElRamahiMD ( View Tweet)
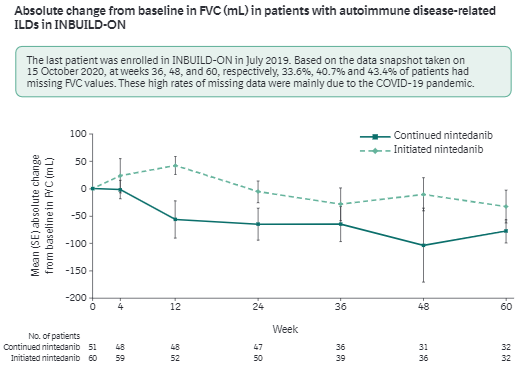
Nintedanib open-label extension study in PF-ILD (progressive fibrosing)
- active arm continuing: similar rate of change as RCT (78 v 77)
- placebo arm initiating: similar as well
Missing data (COVID-19) but effect appears related to exposure
INBUILD-ON #ACR21 ABST0186 @RheumNow https://t.co/hWe7ypbex6
David Liew drdavidliew ( View Tweet)
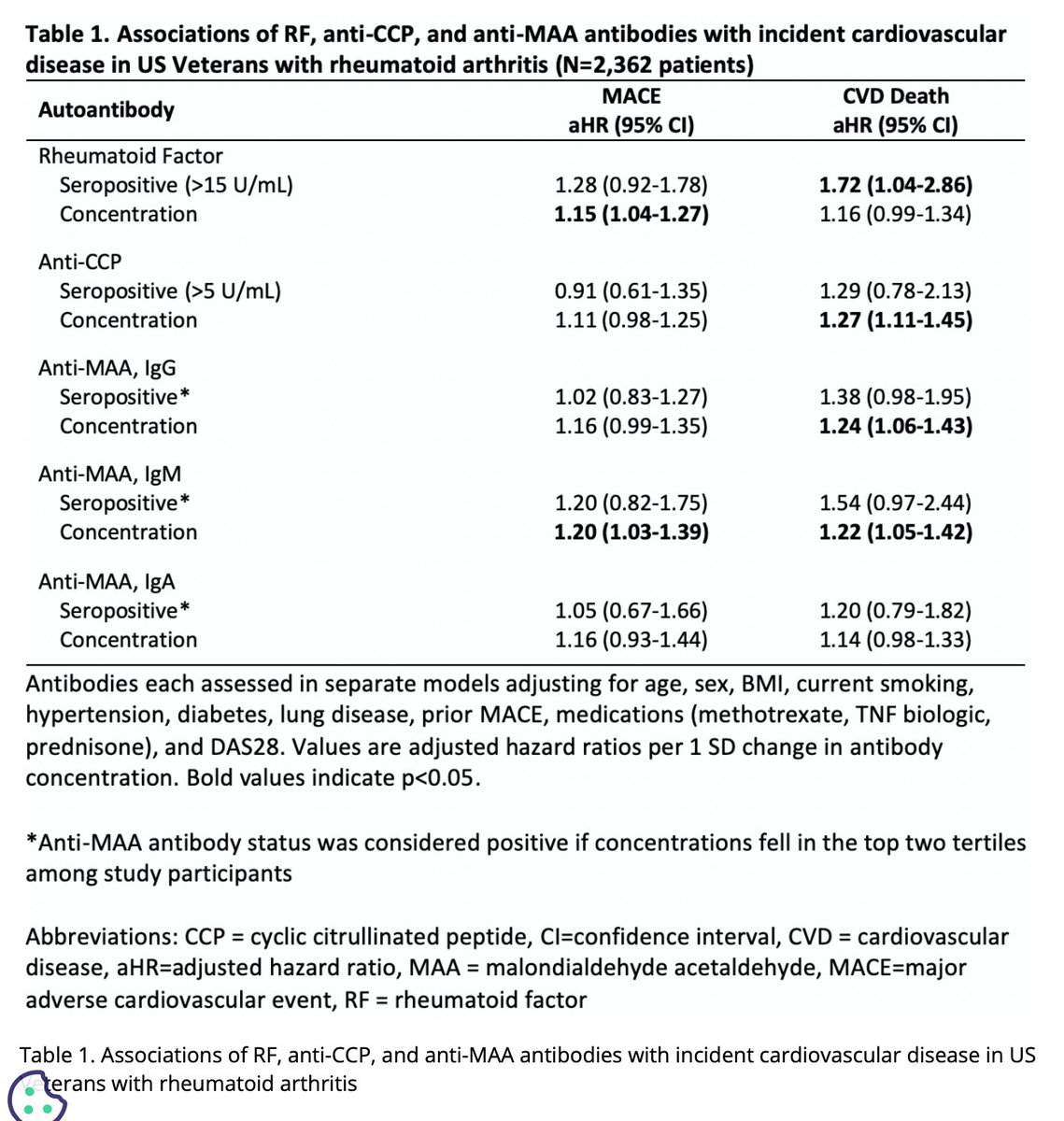
Abst#0269: Autoabs confer risk of incident CVD in US ⚦ veterans w/ RA
⭐︎ MACE risk: ↑ concentrations of RF (HR 1.15) & malondialdehyde acetaldehyde (anti-MAA) (HR 1.2)
⭐︎ CVD Death: ↑ concentrations of CCP (HR 1.27) & anti-MAA
#ACR21 @Rheumnow
https://t.co/UeOz91Bh3P https://t.co/PJwP1H4eNk
Meral K. El Ramahi, MD MeralElRamahiMD ( View Tweet)

How it started How it's going
inspirational talk @DzifaDey, building rheumatology up in Ghana, smart solutions across all domains, and scaling up to help across West Africa
#ACR21 'Challenges & Innovation in Global Rheumatology' session @RheumNow @TheLancetRheum https://t.co/9aG6pes4qy https://t.co/wo6f90ocEP
David Liew drdavidliew ( View Tweet)
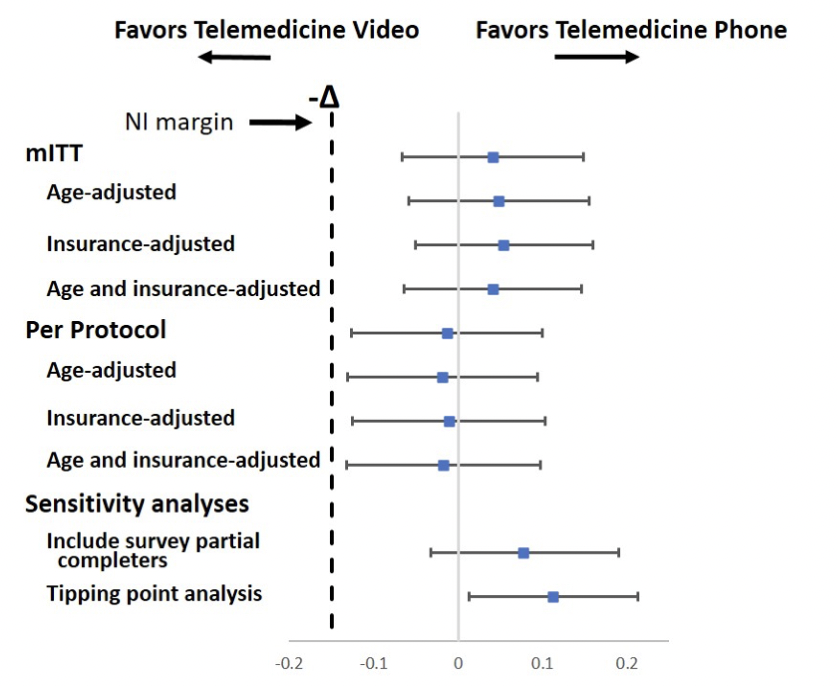
💻 Telemedicine: would you rather have a telephone or a video clinics? Non inferiority RCT shows 80% satisfaction rates and phone-only non-inferior to video visits. I would have expected patients to prefer video visits 🧐 #ACR21 #Abst0112
@RheumNow https://t.co/klgMJ6EthH https://t.co/17ffTAZCfv
Aurelie Najm AurelieRheumo ( View Tweet)

#ACR21 Abst#0269
⭐️TCZ: ⬆️cholesterol, LDL, TG. ⬇️ hsCRP compared to TNFi. No ⬆️ risk of Reynold risk score (RRS)
Take-away: good data on TCZ effect on lipids, but I wouldn't use RRS to calculate CVD risk on med that ⬇️ CRP @Rheumnow https://t.co/zMltktfHkS https://t.co/bwKXiGIi8a
Links:
Eric Dein ericdeinmd ( View Tweet)
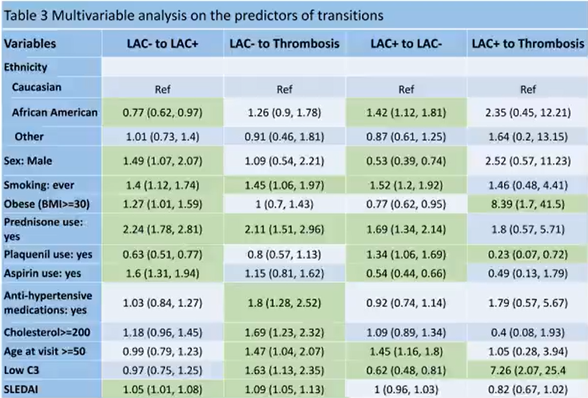
#Abstr0076 #ACR21 Can we predict #thrombosis in #lupus patients? Predictors of serology transition at 5yrs:
✅LAC+ to LAC-:Afro-American,HCQ and normal C3
⛔️LAC+ to 🩸Clot:Obesity, low C3 and not on HCQ
Data need in those with Triple Positivity @RheumNow https://t.co/byeL5NjVUS https://t.co/CchtZir2GN
Md Yuzaiful Md Yusof Yuz6Yusof ( View Tweet)
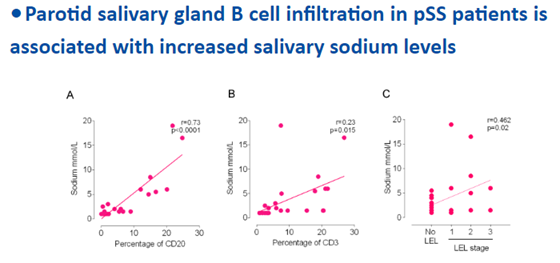
#Abstr0002 #ACR21 What drives salt in saliva in #sjogren? A study suggested Bcell cytokines mediated the epithelial Na+ Channel disruption. A case for targeting Bcell to improve oral health @RheumNow https://t.co/Bx3kBpOIFX https://t.co/YYnC6UoMJY
Md Yuzaiful Md Yusof Yuz6Yusof ( View Tweet)
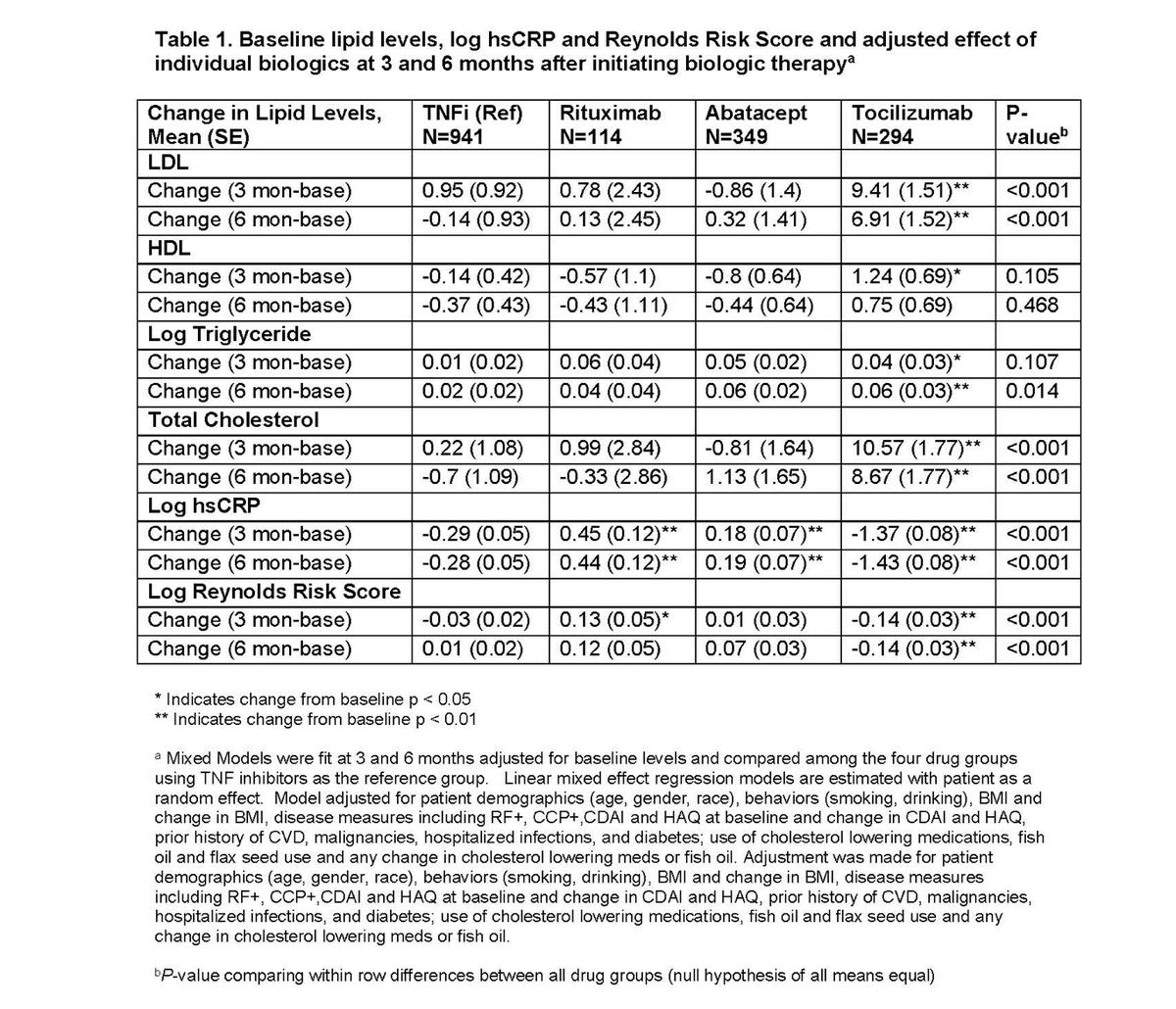
Abst#0268
➡️Do lipids change w/ some bDMARDs in RA? YES!
⭐︎ TCZ: ↑ TC, LDL, TG & ↓ in hsCRP relative to initiating TNFi.
➡️Does this lipid ↑ confer risk for CVD? Not necessarily as it may not be atherogenic per Reynolds Risk Score!
#ACR21 @Rheumnow
https://t.co/Gy62mg6GhZ https://t.co/5uC8Zy0Lbr
Meral K. El Ramahi, MD MeralElRamahiMD ( View Tweet)

Which of the following would you d/c in the 3rd trimester of pregnancy? #ACR21 #Pregnancy @rheumnow
TheDaoIndex KDAO2011 ( View Tweet)

Experts (Drs. M Clowse, Sammaratino) limit mAb TNFi 1 month b/f delivery or extend dosing. But, they do dose again for flares. Risk for infection is low based on the PIANO registry. Postpartum, they restart the meds w/in 1 week (vag delivery), 2 weeks C/S @rheumnow #pregnancy
TheDaoIndex KDAO2011 ( View Tweet)
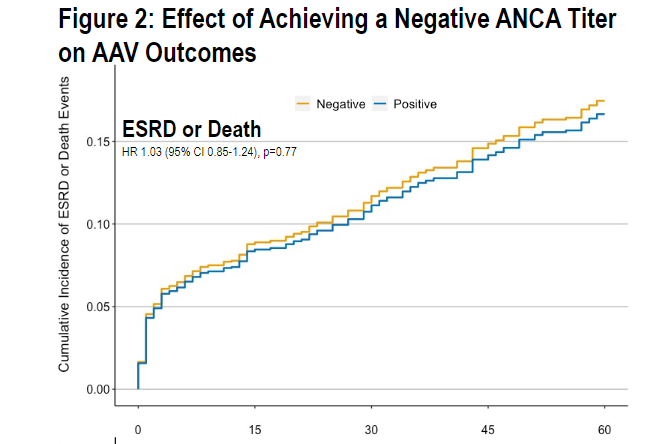
In ANCA-associated vasculitis, does reverse seroconverting (ANCA+➡️ANCA-) make a difference?
relapse - no
ESRD - no
death - no
Don't measure serial ANCAs for prognosis
(no diff MPO/PR3, RTX/CYC)
target trial emulation @BrighamWomens @MGHrheumatology #ACR21 ABST0419 @RheumNow https://t.co/rLkTY0ul8f
David Liew drdavidliew ( View Tweet)
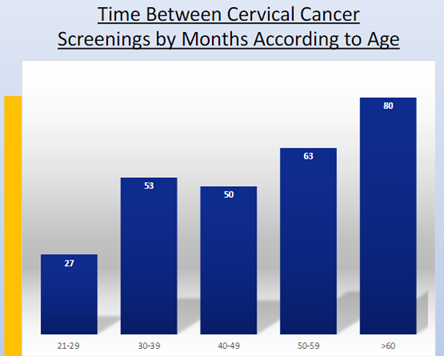
Either we need to review the Yearly PAP cervical screening in SLE women or work hard to improve patient awareness. Suspect the latter as time between tests was over 4 years late 😱 and 2.7% developed cancer #ACR21 #Abstr0133 @RheumNow https://t.co/wm01MfoFjf https://t.co/blD8WafZsE
Md Yuzaiful Md Yusof Yuz6Yusof ( View Tweet)

#ACR21 Abst #0269: VA study of AutoAbs and CVD risk
⭐️Anti-malondialdehyde acetaldehyde (anti-MAA) shown to associate w/ CVD risk in non-RA pt
Findings: RF, CCP, anti-IgG anti-MAA, anti-IgM anti-MAA (not IgA) associated with ⬆️ CVD death
https://t.co/WRBh673u8T @Rheumnow #ACRbest
Links:
Eric Dein ericdeinmd ( View Tweet)


