All News
Methotrexate-Pegloticase Combination in Refractory Gout - MIRROR Study
The MIRROR study, an open-label pilot trial in refractory, uncontrolled gout patients, showed that pegloticase given in combination with methotrexate was safe and effective and maintained responses for 6 months after initiation.
Read ArticleBelimumab Effective in Lupus Nephritis
Dr. Rich Furie has lead a group of investIgators in reporting the significant efficacy of intravenous belimumab when added to standard of care induction therapy in patients with active lupus nephritis. This phase 3, 2-year study included 448 systemic lupus erythematosus patients with biopsy proven lupus nephritis (class III or IV +/- V) patients who required induction therapy and have a UPCR ≥1 g/g.
Read ArticleHas the Risk of Hip Fracture Changed over Time?
Data from the Framingham Heart Study has shown that hip fracture incidence has decreased over time, probably resulting from improved lifestyle changes (less smoking and heavy drinking).
Read ArticleNSAIDs Shows No Advantage over CBT in Knee Osteoarthritis
JAMA Internal Medicine reports on a controlled clinical trial of knee osteoarthritis patients wherein meloxicam responses were not significantly or clinically superior to placebo or CBT (after placebo).
Read ArticleCapsaicin Injection Improves Knee OA Pain
Highly purified synthetic trans-capsaicin (CNTX‐4975) injected directly into the joint reduced pain in chronic knee osteoarthritis (OA), data from the open-label phase III VICTORY-3 trial showed.
Read ArticleUpdated List of Drugs Inducing Lupus
Drugs inducing lupus may account for nearly 30% of all subacute cutaneous lupus erythematosus (CLE) and up to 15% of systemic lupus erythematosus (SLE) cases. Moreover there are over 100 drugs capable of inducing drug-induced lupus erythematosus.
Read ArticleComorbidities Impact Disease Activity in Spondyloarthritis
An analysis of a large population-based cohort of axial spondyloarthritis (axSpA) patients shows that comorbidities are common and are associated with higher disease activity and higher levels of functional impairment.
Read ArticleRheumNow Podcast - ACR Reproductive Health Slides (9-11-20)
Dr. Jack Cush reviews the 2020 ACR Reproductive Health Guidelines presented all week here on RheumNow.
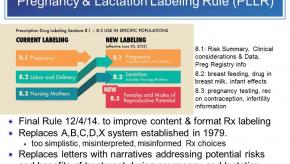
Read ArticleICYMI - The Pregnancy & Lactation Labeling Rule Explained
A recent article from Healio.com reviewed the FDA’s Pregnancy and Lactation Labeling Rule (PLLR) that became effective June 30th.
Read ArticleACR 2020 Reproductive Guidelines - Medication Use with Pregnancy
The American College of Rheumatology (ACR) has published guidelines to manage reproductive health issues in rheumatic disease (RMD) patients before, during and after pregnancy.
Read ArticleRheumNow Podcast – To Celiac Test or Not? (9.4.20)
Dr Jack Cush reviews the news, journal articles and takes a few Back Talk questions this week:
Read ArticleTreatment Options for Extraintestinal IBD
Extraintestinal manifestations (EIMs) frequently accompany inflammatory bowel disease, often augmenting morbidity and mortality are associated with disease activity and may increase the need for surgery or treatment escalation. A recent review in GUT details the treatment options for patients with EIM.
Read ArticleOlder Lupus Patients Can Stop HCQ Without Increasing Flare Risk
Since patients with SLE have longer life expectancies in the modern era, they may be at elevated risk for HCQ adverse events, including maculopathy and cardiomyopathy. Therefore, Peter M. Izmirly, MD, of New York University School of Medicine in New York City, and colleagues assessed whether older SLE patients could safely discontinue HCQ.
Read ArticleEarly Cardiovascular Benefits to DMARD Treatment in Rheumatoid Arthritis
ARD has published a report showing that patients with early rheumatoid arthritis have cardiovascular disease that is modifiable with conventional and aggressive therapy and that it did not matter if first line DMARD therapy was with a tumour necrosis factor-inhibitor or methotrexate.
Read ArticleCOVID and the Multisystem Inflammatory Syndrome in Children
Lancet Infectious Disease has reviewed the epidemiology, causes, clinical features, and current treatment for the multisystem inflammatory syndrome in children (MIS-C) and adolescents associated with COVID-19, noting severe organ damage can occur pediatric COVID-19 patients who are severely ill.&
Read ArticleCDC: COVID Seroprevalence in Health Care Workers
CDC reports that gmong 3,248 healthcare personnel (HCP) observed, 6% had antibody evidence of previous SARS-CoV-2 infection; 29% of personnel with SARS-CoV-2 antibodies were asymptomatic in the preceding months, and 69% had not previously received a diagnosis of SARS-CoV-2 infection.
Read ArticleSleep, Fatigue and Depression in Psoriatic Arthritis
Comorbidities are highly prevalent in psoriatic disease. A recent study in Arthritis Research & Therapy suggests a high prevalence of sleep disturbances, fatigue, and anxiety/depression in psoriatic arthritis (PsA) that is unrelated to skin disease musculoskeletal (MSK) man
Read ArticleRheumNow Podcast – Rheum Lives Matter (8.28.20)
Dr. Jack Cush reviews the news and journal articles from the past week. Of mice and men in cartilage, the efficacy of JAK inhibitors in patients who are having skin problems, nrAxSpa has its own diagnostic code, metformin, talking about Back Talk, plus so much more.
Read Article
Links:

Links:
Dr. John Cush RheumNow ( View Tweet)