All News
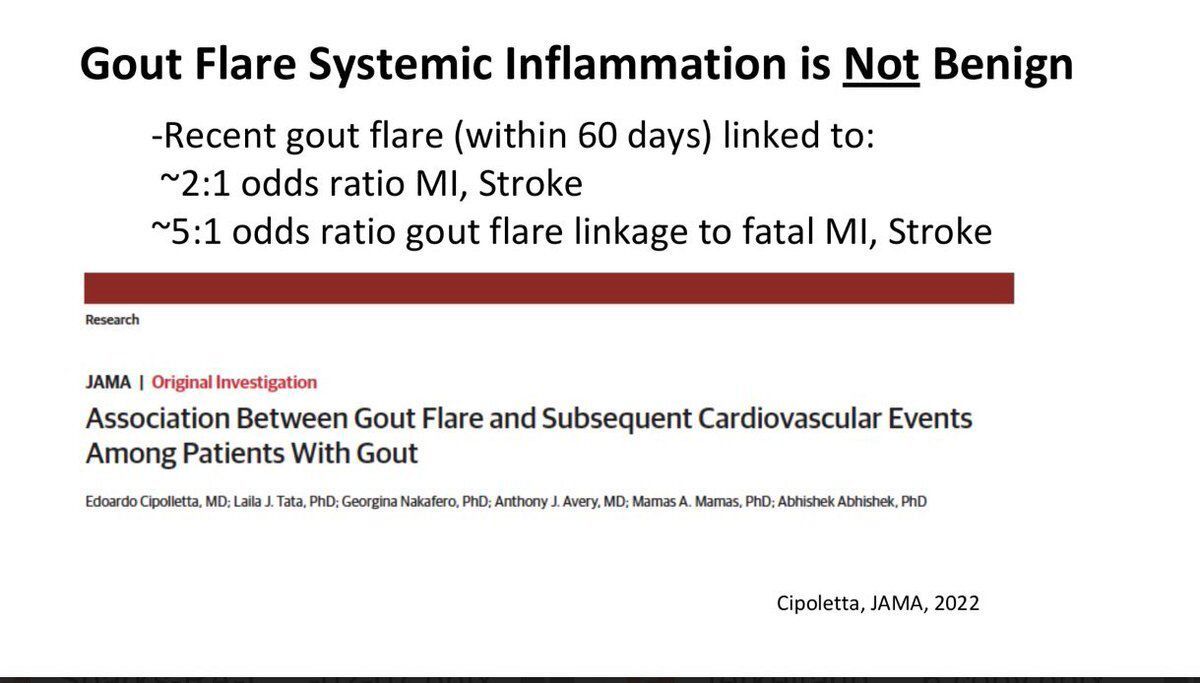
Treat-to-Target and Cardiovascular Benefits in Gout
A new user cohort study of 109 504 gout patients, achieving a serum urate level less than 6 mg/dL, was associated with a significantly lower risk of cardiovascular events.
Read ArticleDisease Modification, Disparities and the Next Therapeutic Frontier in Gout
Gout management has entered what Dr. Robert Terkeltaub MD from UC San Diego described as its “disease-modifying era,” during his talk at RheumNow Live 2026. In a recent comprehensive review of the past, present, and future of gout therapy, the central message was clear: “We can really apply disease modification to gout based on prospective, randomized controlled trials.”
Read ArticleANA Pollution (2.06.2026)
Dr. Jack Cush reviews the news, journal articles and regulatory news from this past week on RheumNow.com
Read ArticleSGLT2 Inhibitors in Gout - Better Outcomes, Fewer Meds
Natalie McCormick, PhD, of the Rheumatology and Allergy Clinical Epidemiology Research (RACER) Center within the Division of Rheumatology in the Mass General Brigham Department of Medicine
Read ArticleRheumNow Live Preview (1.30.2026)
Dr. Jack Cush reviews the news and journal reports from RheumNow.com - and the top 5 reasons to attend RheumNow Live 2026, which is one week away.
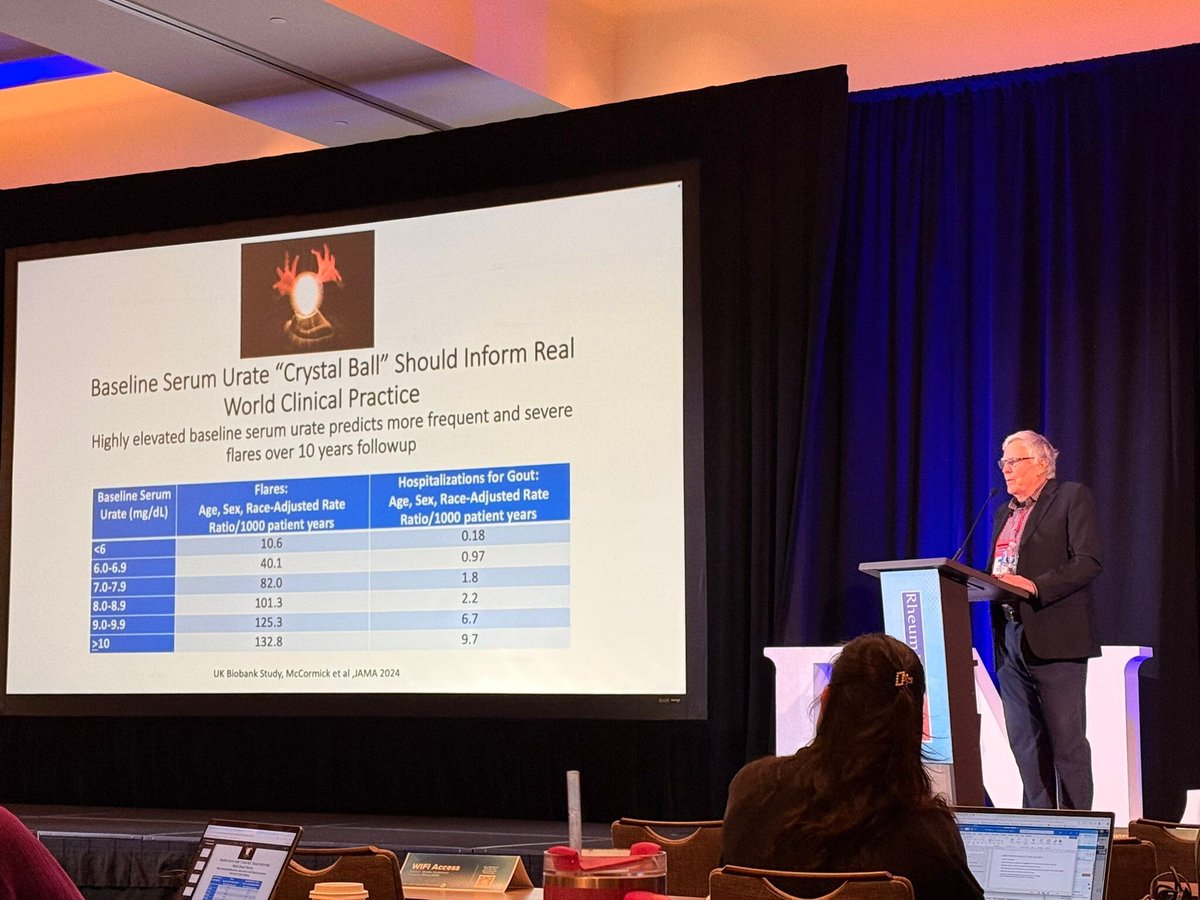
Read ArticleTreat-to-Target in Gout and Cardiovascular Outcomes
A 5 year JAMA study shows that a treat-to-target (serum urate < 6 mg/dL) study finds that effective urate-lowering treatment (ULT) results in a significantly reduced cardiovascular risk in patients with gout.
2026 Resolutions (1.9.2026)
Dr. Jack Cush reviews the news, announcements and journal articles from this past week on RheumNow.com. More on Variable bendability, a better way to treat RA, and a novel advance for GLP1a in PsA; and 2026 Resolutions!
Read ArticleBest of 2025: Distinguishing Septic and Gouty Arthritis
A single center, retrospective review of patients undergoing knee joint fluid aspirations for presumed crystalline arthritis (CA) showed that synovial WBC may provide a useful diagnostic marker for SA with an optimal threshold of 50,000 cells/mm3.
Read ArticleReefer Madness (12.12.2025)
Dr. Jack Cush reviews the news, journal and regulatory reports from this past week on RheumNow.com. B cell drugs in SLE and ITP, biomarkers in GCA & PSS and great videos by APPs.
Read ArticleI Can't Believe it's Not Seronegative Rheumatoid Arthritis?!
In rheumatology, we're no stranger to uncertainty. In any diagnosis of seronegative rheumatoid arthritis, it's always our invitation to reconsider the diagnosis.
Read Article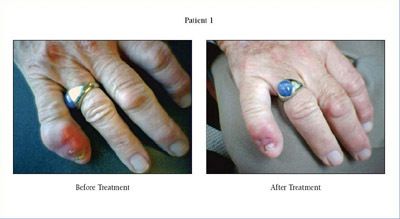


Links:
QD Clinic: Tophi Tales: Management of Uncontrolled Gout Daric Mueller, PA-C and Andrew Sulich, MD, St. Clair Shores, MI, present this QD Clinic: Tophi Tales: Management of Uncontrolled Gout. This QD Clinic is presented as part of RheumNow's "Mission: APP Partners in Care" https://t.co/7JTbC0223I
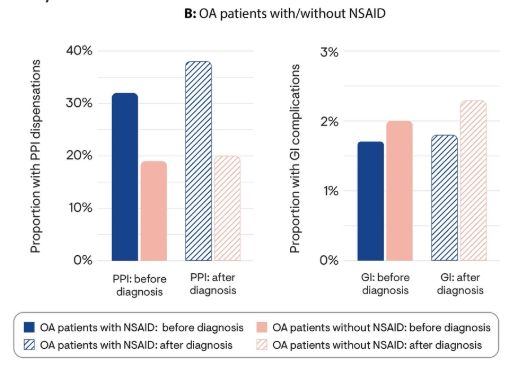

Dr. John Cush RheumNow ( View Tweet)
Links: