Advances in RA-ILD Save
Dr. Jeffrey Sparks gave a state of the art update on Advances in RA-ILD, many of which he and his group have played a big part in, on Saturday at RNL26.
Dr. Sparks began by presenting some recent epidemiologic data reminding us of the scale of the problem. RA-ILD is common, and probably more common than most of us intuitively believe. A recent meta-analysis showed a pooled prevalence of 11%, and an update of the Olmstead County data showing a cumulative incidence of 15.3% over 20 years. UIP is the predominant radiologic pattern in RA-ILD but it is by no means exclusively UIP and fibrotic disease we see, with 40-50% having more inflammatory patterns which has implications for treatment choices – we should not lump all RA-ILD together as a predominantly fibrotic process.
We transitioned to data from the BRASS study demonstrating the role that RA disease activity plays in risk of RA-ILD. There is a strong relationship evident here. DAS28 moderate disease activity having a multivariable HR of 2.08 and high disease activity a multivariable HR of 3.48 for RA-ILD. There also appears to be a linear relationship, at least above a certain threshold with a multivariable HR of 1.35 for each point increase in DAS28.
Screening for RA-ILD is a controversial area for many reasons. There is a question of feasibility and cost, due to the poor performance of CXR, screening usually involves high resolution CT thorax and pulmonary function tests. It is not pragmatic in current health care systems to screen all RA patients. Dr. Sparks presented the ACR/Chest 2023 guidelines suggesting possibly screening in RA patients with risk factors and also described risk factor scores to try and identify the optimum screening population. This reduces the numbers screened from the total population but is still a very significant population to screen. There are real risks with an increased proportion of screening of not just radiation exposure and burden to patients, but also of over-diagnosis and over-treatment, as well as the problems of incidental findings and testing cascades. I don’t think we know what the best approach to screening for RA-ILD is based on current evidence. The defensive medicine based tendency of “if in doubt, screen” may not be the optimum strategy.
We went on to medications. As always methotrexate first. MTX pneumonitis does occur but is rare – 0.3% of methotrexate treated patients vs <0.1% of patients not on methotrexate in the 4186 patient CIRT RCT. There has been yet another meta-analysis looking at MTX and risk of incident RA-ILD, and yet another result showing no increased risk. In this case an OR of 0.49, consistent with a 51% decreased risk of RA-ILD with methotrexate use and tying in with the evidence around disease activity (rather than medications) driving ILD risk.
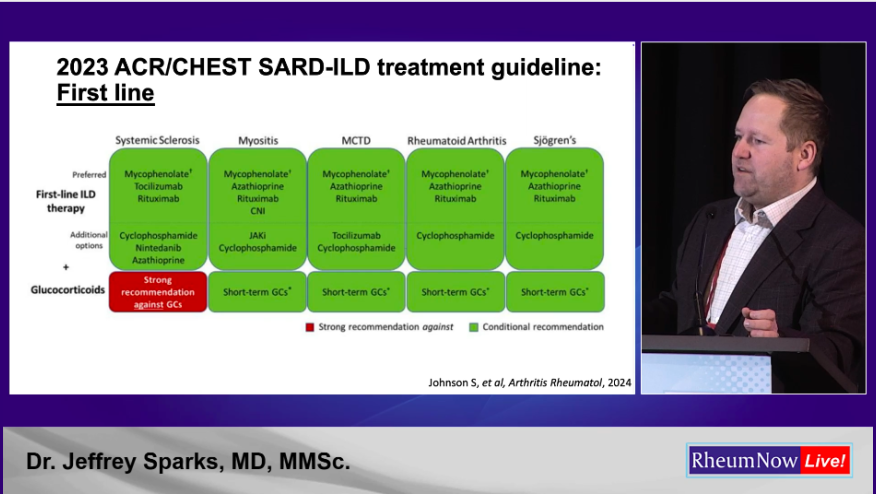
Dr. Sparks went on to present the ACR/Chest 2023 and EULAR/ERS 2025 guidelines, specifically those that addressed RA-ILD. Regular readers will know of my views on the ACR/Chest guidelines. In contrast I think the EULAR/ERS guidelines are excellent and closely match my own clinical practice. (I was not involved in either set of guidelines). The differences between the guidelines reflect the lack of evidence in this space, something that should improve given the active research efforts in this space.
Dr. Sparks then presented the recent data on nerandomilast. This drug entered the anti-fibrotic space but has additional anti-inflammatory and immunomodulating functions, not surprising in a relative of apremilast. This drug demonstrates additional benefits in terms of pulmonary function test preservation in those already on nintedanib. But what really made people sit up and take notice is the significant mortality benefits demonstrated, with HR of 0.48 to 0.60.
Dr Sparks showed data from Scott Matson and colleagues demonstrating that immunosuppression of RA-ILD, agnostic to the agent utilised, leads to arrest of pulmonary function test decline and subsequent stabilization. This is a major point against those that argue that RA-ILD should solely be treated with anti-fibrotic agents, similar to idiopathic pulmonary fibrosis.
Finally and thought-provokingly he presented a target trial emulation study from his own group. This showed better outcomes for RA-ILD in those treated with abatacept or JAK inhibitors compared to rituximab. Outcomes for TNF inhibitors and IL-6 inhibitors were similar to rituximab. This challenges the firm, but weakly supported by evidence, belief that rituximab is the agent of choice in this patient cohort.
Join The Discussion
Interesting last paragraph Richard. Anyone looking at IV Abacept plus jaki combo in truly difficult to treat RA from an ILD perspective?
Thanks Sinead. Not that I am aware of, but hypothetically attractive and would be very interesting to see some data.










If you are a health practitioner, you may Login/Register to comment.
Due to the nature of these comment forums, only health practitioners are allowed to comment at this time.