Tweet Content
203 Rheumatology Clinical trials .gov; after 30 mos 17% are unpublished. Published were more likely to be Phase 3 (57% v 29%) or have +primary outcome (65% vs 26%). Unpub authors cite ongoing preparation, sponsor issues, negative results https://t.co/zSxt0W9ZhB https://t.co/k9rCUSel3e
Links
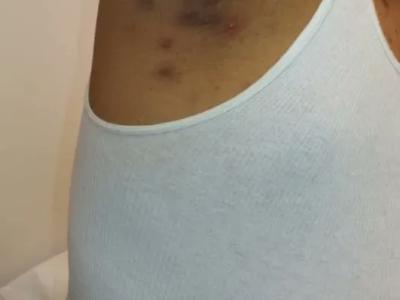
Bimekizumab in Hidradenitis Supprativa (HS) - results of 2 phase III BE HEARD I & II trials show Week 16 50% HS Res
Tweet Content
Bimekizumab in Hidradenitis Supprativa (HS) - results of 2 phase III BE HEARD I & II trials show Week 16 50% HS Responses to be 48% and 52% (vs PBO 29% and 32%). >75% maintained response out to wk 48; at wk 48, a 75% HS response seen in 55%. https://t.co/ZxJylP7IUk https://t.co/Kmx1dkHycM
Links
The EMA Committee for Medicinal Products for Human Use (CHMP) adopted a positive opinion, regarding the marketing author
Tweet Content
The EMA Committee for Medicinal Products for Human Use (CHMP) adopted a positive opinion, regarding the marketing authorisation of deucravacitinib (Sotyktu) in the treatment of moderate to severe psoriasis based on the POETYK PSO-1 & POETYK PSO-2 RCTs https://t.co/KBIYSJVFqu
. https://t.co/nzuHh6v0ua
Links
Frunevetmab (Solensia), is a cat-specific mAb against nerve growth factor was FDA approved, for use in CATS, to treat th
Tweet Content
Frunevetmab (Solensia), is a cat-specific mAb against nerve growth factor was FDA approved, for use in CATS, to treat the pain of osteoarthritis. CATS! https://t.co/mqmQ7jzbpj https://t.co/9FrIOAYJwA
Links
Abbvie has completed a phase II upadacitinib vs combo elsubrutinib/upadacitinib ( ABBV-599) in active #SLE - M19-130 (SL
Tweet Content
Abbvie has completed a phase II upadacitinib vs combo elsubrutinib/upadacitinib ( ABBV-599) in active #SLE - M19-130 (SLEek) Phase 2 trial met the primary endpoint SRI-4 response and pred dose<=10 mg qd. No new safety signals - Phase III trial planned https://t.co/1ZiRX3jUGV https://t.co/k0a54QJ9Hi
Links
2022 EULAR Recommendations for ANCA-associated Vasculitis EULAR has published the 2022 update on recommendations for th
Tweet Content
2022 EULAR Recommendations for ANCA-associated Vasculitis
EULAR has published the 2022 update on recommendations for the management of antineutrophil cytoplasmic antibody (ANCA)-associated vasculitis (AAV).
https://t.co/kX3dg9DGuw https://t.co/b27CX6Bn0h
Links
LAVLI - A New Autoinflammatory DisorderNIH researchers have have described a novel autoinflammatory disorder called "L
Tweet Content
LAVLI - A New Autoinflammatory Disorder NIH researchers have have described a novel autoinflammatory disorder called "Lyn kinase-associated vasculopathy and liver fibrosis" (LAVLI), based on a mutation in the LYN gene. https://t.co/VWs0YuF4LD https://t.co/7ydA3bb82j
Links