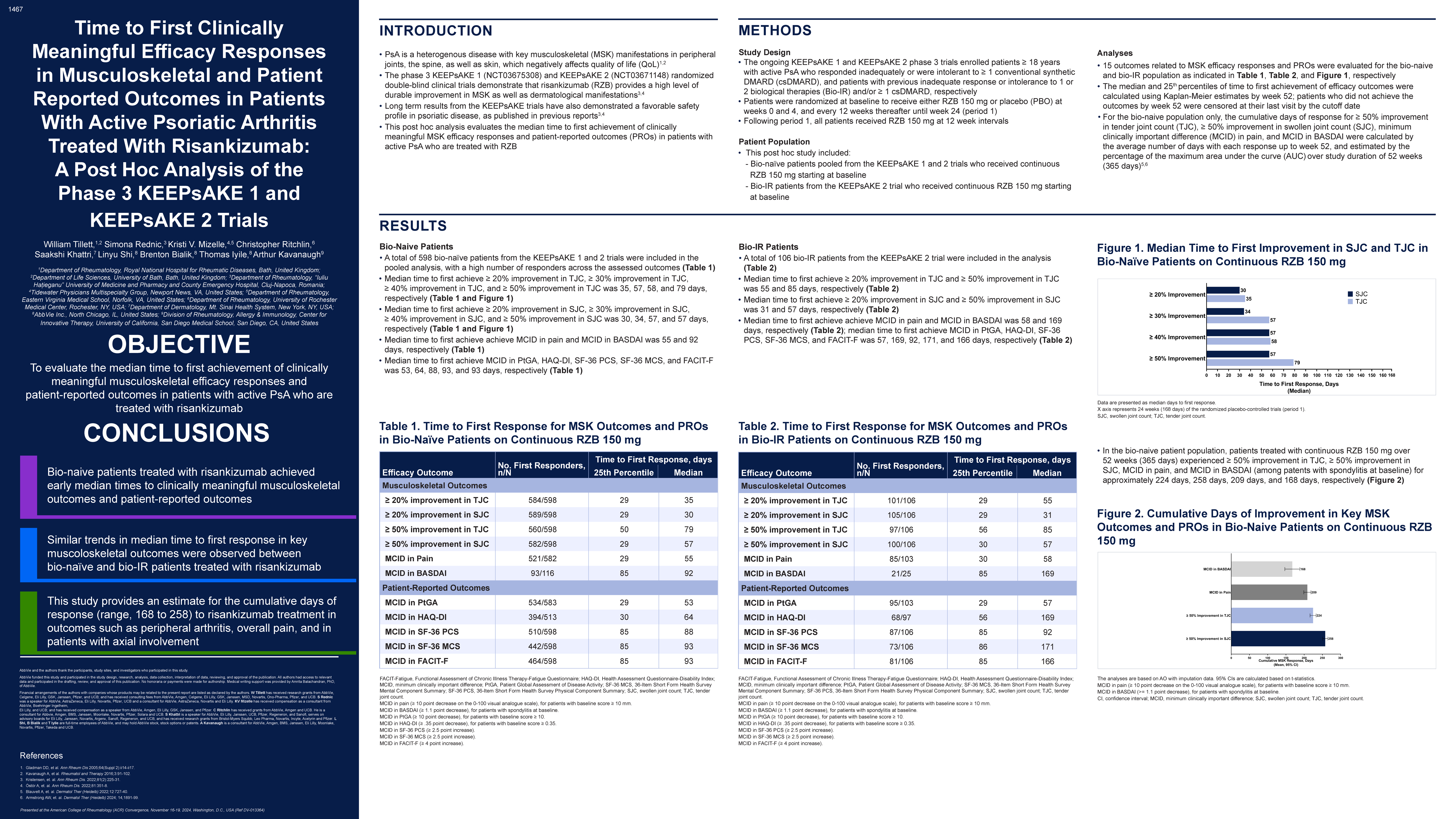
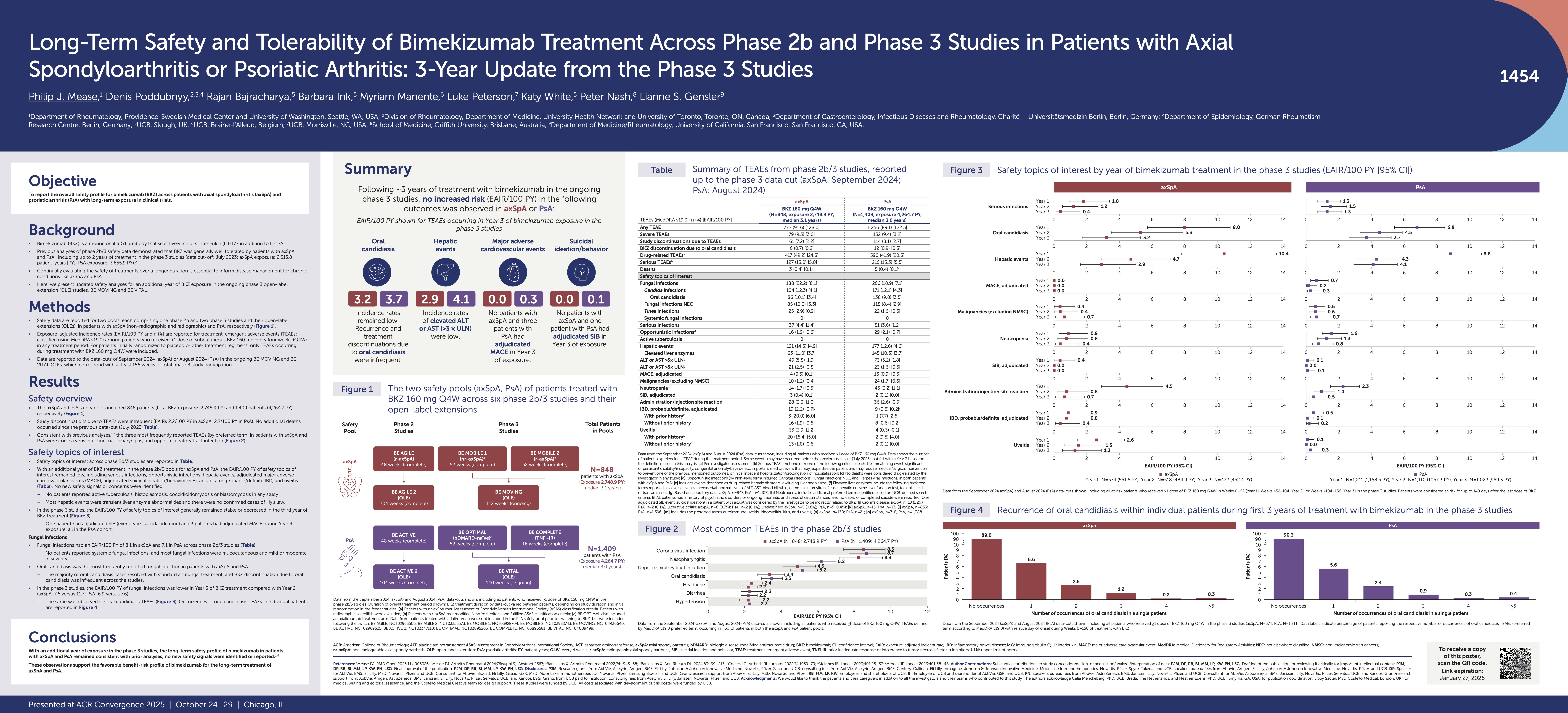
RISANKIZUMAB-RZAA INDICATIONS AND IMPORTANT SAFETY CONSIDERATIONS
INDICATIONS
Risankizumab is indicated for the treatment of moderate to severe plaque psoriasis in adults who are candidates for systemic therapy or phototherapy.
Risankizumab is indicated for the treatment of active psoriatic arthritis in adults.
IMPORTANT SAFETY CONSIDERATIONS
Risankizumab is contraindicated in patients with a history of serious hypersensitivity reaction to risankizumab or any of the excipients. Serious hypersensitivity reactions, including anaphylaxis, may occur. If a serious hypersensitivity reaction occurs, discontinue risankizumab and initiate appropriate therapy immediately. Risankizumab may increase the risk of infections. Instruct patients to seek medical advice if signs or symptoms of clinically important infection occur. If such an infection develops, discontinue risankizumab until the infection resolves. Evaluate patients for tuberculosis infection prior to initiating treatment with risankizumab. Avoid use of live vaccines in patients treated with risankizumab. The most common adverse reactions (≥1%) are upper respiratory infections, headache, fatigue, injection site reactions, and tinea infections.
Review accompanying risankizumab-rzaa full Prescribing Information for additional information, visit rxabbvie.com or contact AbbVie Medical Information at 1-800-633-9110.