All News
ANA Pollution (2.06.2026)
Dr. Jack Cush reviews the news, journal articles and regulatory news from this past week on RheumNow.com
Read ArticleThe 2025 Rheumatology Year in Review
The year 2025 presented numerous advances in rheumatology and related inflammatory and autoimmune disorders ranging from several new groundbreaking FDA approvals/indications, drug developments, game-changing guidelines and practices that will impact patient care for rheumatic diseases.
Read ArticleAdvanced Practitioner Biologic Prescriptions for Psoriasis
Advanced practice clinicians (APCs; nurse practitioners and physician assistants) deliver a large share of US dermatologic care, accounting for 37% of clinicians and 27% of dermatology visits by 2020. A current JAMA Dermatology reports APC drug spending trends in dermatology, with a focus on specialty medications (eg, biologics).
Read ArticleAPEX Study: Inhibition of structural damage progression with Guselkumab in Psoriatic Arthritis
Guselkumab, a human IL-23 inhibitor, was evaluated in patients with active psoriatic arthritis (PsA) and shown to have both clinical and radiographic benefits at weeks 24 and 48.
Read ArticleACR 2025 Rheumatology Round Up
Drs. Jack Cush & Arthur Kavanaugh, two of rheumatology’s most trusted voices, provide a breakdown of the latest breakthroughs and hottest topics in rheumatology from the 2025 ACR Convergence meeting in Chicago.
Read ArticleACR25 Best Abstracts - Day 4
Here's the last installment of our "ACR Best" abstracts as chosen by the RheumNow faculty. Most of these were from the final, day 4, but a few were noteworthy holdovers from day 3. Enjoy!
Read ArticleIL-23 Blockade Goes Oral
A new player is entering the IL-23 arena — and it’s a tablet! Icotrokinra (ICO), a first-in-class peptide that binds and blocks the interleukin-23 receptor (IL-23R), is showing encouraging efficacy and safety across a range of psoriasis (PSO) and psoriatic disease (PsD) studies.
Read ArticleEating Crow (10.10.2025)
Dr. Jack Cush reviews the news, FDA approvals, and journal articles. In this episode: HMGCR Abs, FDA approvals and Cush eats crow.
Read ArticleGuselkumab FDA Approved for Pediatric Psoriasis and Psoriatic Arthritis
FDA announced yesterday that guselkumab (Tremfya) is approved for use in pediatric patients moderate to severe plaque psoriasis (PsO) or active psoriatic arthritis (PsA) in children six years and older (weighing at least 40 kg).
Read ArticleSafety of Combination Targeted Therapies in Psoriatic Arthritis
Treating psoriatic arthritis (PsA) patients with more than one targeted therapy appears to be just fine, with no increased risk of serious infections, insurance claims data indicate.
Read ArticleBiologic Switching in Psoriasis
A systematic review and meta-analysis found that biologic switching was effective in a significant number of psoriasis patients; but there may be a price to pay.
Read Article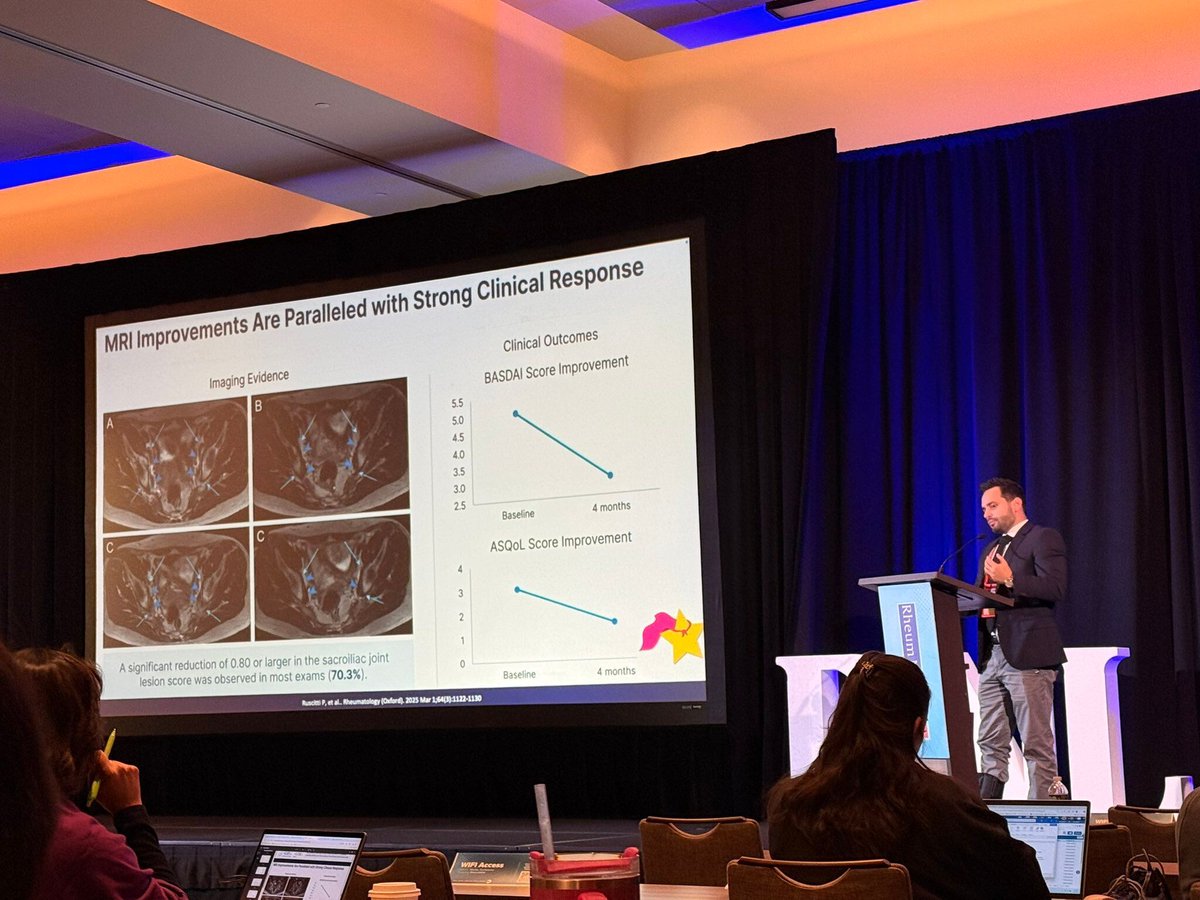



Links:
Nouf Al hemmadi NoufAhmedAlham2 ( View Tweet)


Links:
Links:

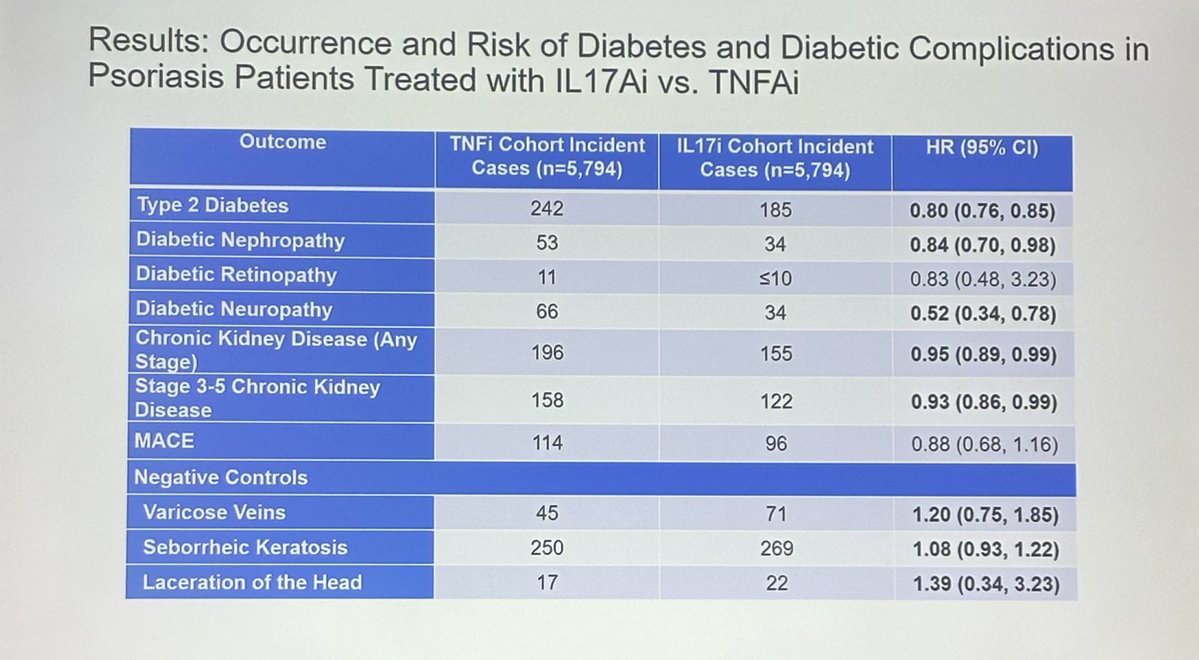
Dr. John Cush RheumNow ( View Tweet)