All News
Adverse Events With Rheum Biologics Rise With Age
Among patients with rheumatic diseases, age and female sex were important factors associated with the development of a first adverse event after initiating biologic treatment, Spanish researchers reported.
Read ArticleHydroxychloroquine Fails as Postexposure Prophylaxis for Covid-19
Add this to the list of hydroxychloroquine (HCQ) letdowns in managing COVID-19 treatment or risk - prophylaxis with HCQ fails to prevent COVID-19 infection in those exposed to the SARS-CoV-2 virus.
NEJM has reported a prospective, randomized, double-blind, placebo-controlled trial from the US and Canada wherein adults with household or occupational exposure to someone with confirmed Covid-19 were treated with either placebo or HCQ within 4 days of exposure.
Upside Down with Tocilizumab in COVID-19
Several recent reports offer conflicting views on the potential benefits and adverse outcomes of IL-6 inhibition with tocilizumab (TCZ) therapy in patients with severe COVID-19 infection.
An Italian study grabbed the headlines yesterday with a press release stating TCZ did not improve respiratory symptoms, ICU admissions or mortality rates when given to 126 COVID patients with early disease. The study, supported by the Italian Medicines Agency (Aifa), stopped enrollment (about 1/3 of projections) after an interim analysis found insufficient evidence that TCZ would be effective. This report has not been published or undergone critical review.
RheumNow Podcast – Enough Already with Weaning (6.19.20)
Dr. Jack Cush reviews the news and journal reports from the past week on RheumNow.com.
Read ArticleCosentyx FDA Approval for Non-Radiographic Axial Spondyloarthritis
Yesterday the FDA approved secukinumab (Cosentyx) for the treatment of patients with active non-radiographic axial spondyloarthritis (nr-axSpA). There are now three agents that are FDA approved for nr-axSpA: certolizumab, ixekizumab and now, secukinumab.
Read ArticleVoclosporin Beats Standard of Care in Lupus Nephritis
The high potency calcineurin inhibitor voclosporin plus standard of care was superior to standard of care alone in a phase III study of lupus nephritis known as AURORA.
Read ArticleCanakinumab First FDA-Approved Therapy for Adult-Onset Still's Disease
Yesterday, the FDA approved canakinumab (Ilaris) as treatment for adult-onset Still's disease (AOSD), the first ever FDA approved drug for AOSD.
Canakinumab, an interleukin-1 inhibitor, was FDA approved in 2013 for use children (aged 2 years and older) with systemic juvenile idiopathic arthritis and has since been approved for use in other periodic fever disorders (e.g., FMF, CAPS, TRAPS, Hyper IgD syndrome).

Links:
Dr. John Cush RheumNow ( View Tweet)

Links:
Dr. John Cush RheumNow ( View Tweet)

Links:
Dr. John Cush RheumNow ( View Tweet)

Links:
Dr. John Cush RheumNow ( View Tweet)

Links:
Dr. John Cush RheumNow ( View Tweet)

Links:
Dr. John Cush RheumNow ( View Tweet)
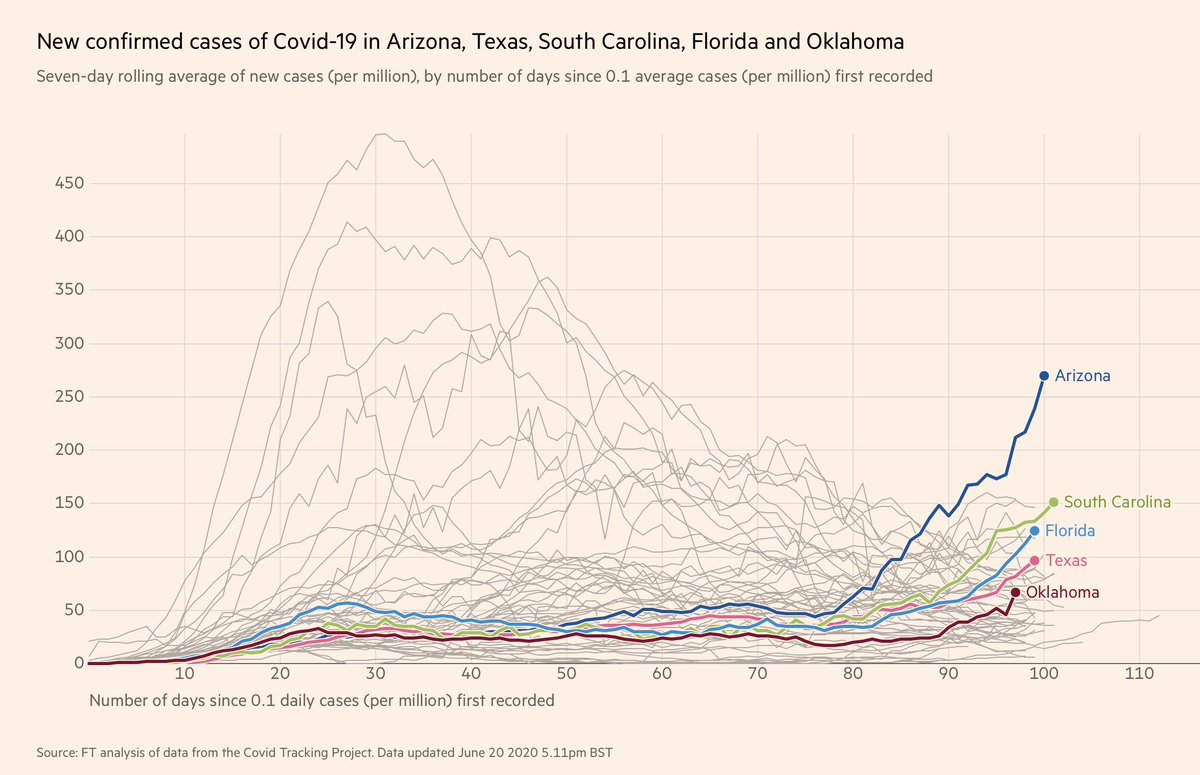

Dr. John Cush RheumNow ( View Tweet)

Dr. John Cush RheumNow ( View Tweet)

Links:
Dr. John Cush RheumNow ( View Tweet)

Links:
Dr. John Cush RheumNow ( View Tweet)

Links:
Dr. John Cush RheumNow ( View Tweet)

Links:
Dr. John Cush RheumNow ( View Tweet)