All News
Upadacitinib Effective in Phase 3 Psoriatic Arthritis Study
Abbvie has announced top line results of their SELECT-PSA trial of upadacitinib (UPA), wherein both the 15 and 30 mg doses met the primary endpoint of ACR20 response at week 12 and demonstrated radiographic inhibition at week 24.
Read ArticleRheumNow Podcast- Best Biologics (2.7.20)
Dr Jack Cush reviews the news and journal articles from the past week on RheumNow.com
Read ArticleBariatric Weight Loss Fails to Alter RA Risk
Obesity has been shown to be a risk factor for the onset of rheumatoid arthritis (RA) and also shown to affect outcomes by impairing responses to many DMARD therapies. A Swiss study of RA patients undergoing bariatric surgery failed to show that bariatric surgery and weight loss had any effect on the incidence of RA.
Read ArticleWhich Biologics are Best in Psoriasis
A metanalysis of phase II, III and IV trials in moderate to severe plaque psoriasis suggests comparative efficacy biologic treatments, but that that brodalumab, guselkumab, ixekizumab, and risankizumab-rzaa were shown to have the past skin (PASI) response rates.
Read ArticleAspirin after Hip or Knee Arthoplasty
JAMA Internal Medicine has reported that use of low dose aspirin for venous thromboembolism (VTE) prophylaxis after total hip and knee replacement is equal in efficacy to other anticoagulants.
Read ArticleStart with Anti-TNF in RA? Not So Fast
The suggestion to hit rheumatoid arthritis (RA) early and hard with biologic therapies itself took a hit in a new study.
Read ArticleLimited Advantage to Very Early vs. Delayed Etanercept in RA
The VEDERA study sought to confirm whether the very early introduction of first-line etanercept+methotrexate (ETN+MTX) was superior to treat-to-target MTX (MTX-TT) in patients with early RA.
Read ArticleEULAR Recommendations on Sjögren’s Syndrome
The European League Against Rheumatism (EULAR) has established an international collaborative group (EULAR SS Task Force) to develop the first EULAR evidence and consensus-based recommendations for the management of patients with Sjogens syndrome (SjS).
Read ArticleHalf of Opioids Rx Come from 1% of MDs
The BMJ reports that while most US providers are cautious in their prescribing, half of opioid prescriptions are written by 1% of providers.
Between 2003 and 2017, there was an annual average of 669495 providers prescribing 8.9 million opioid prescriptions.
Read ArticleRheumNow Podcast- 2019 EULAR RA Guidelines (1.31.20)
Dr. Jack Cush reviews the news and journal articles from the past week on RheumNow.com.
Read ArticleHormone Therapy for Postmenopausal Women
The NEJM weighs in on the problem of post-menopausal osteoporosis (OP) and tackles the use of hormonal therapy.
The decline in estrogen after menopause may increase risks for osteoporosis, cardiovascular disease, and cognitive decline. The use of hormone replacement therapy (HRT) to obviate these issues may be primarily driven by hot flashes in postmenopausal women.1
Who may benefit from hormone therapy among postmenopausal women?
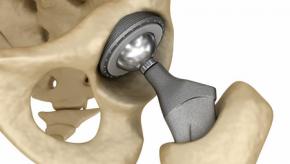
Knee Replacements Last 25 Years
UK registry reports that greater than 80% of total knee replacements can last for 25 years.
The outcomes regarding the duration and durability of knee arthroplasties is sketchy, with many orthopedists projecting a 15 to 20 year survivial. Hence the need for an appraisal of the data.
Read Article
NEJM NEJM ( View Tweet)

Dr. John Cush RheumNow ( View Tweet)
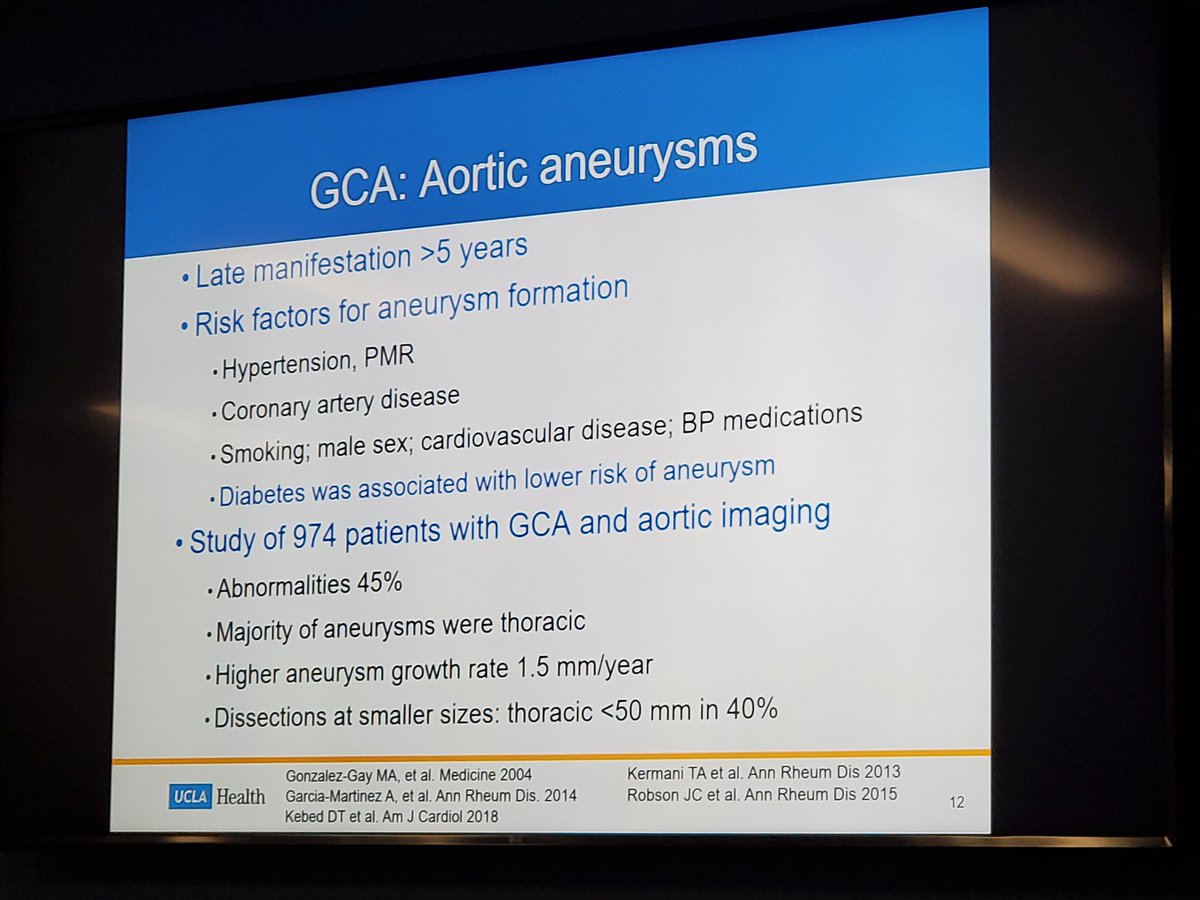

Dr. John Cush RheumNow ( View Tweet)



Dr. John Cush RheumNow ( View Tweet)