All News
Organ Involvement with Behçet’s
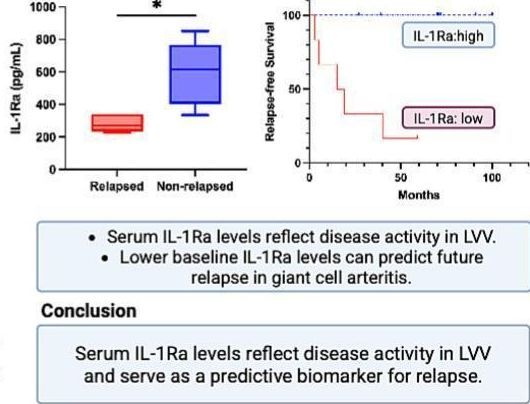
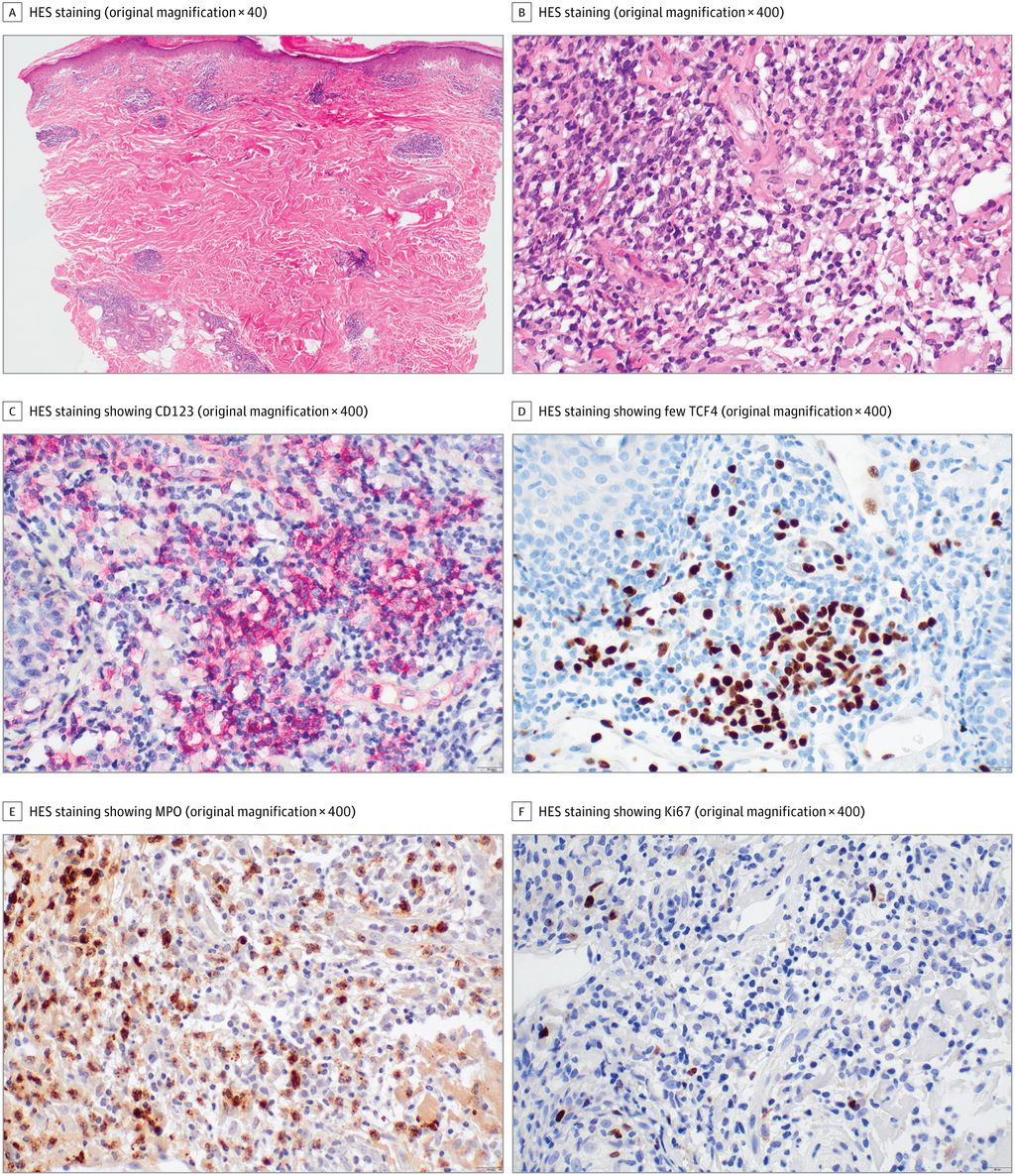
A review of patient data from the International AutoInflammatory Disease Alliance (AIDA) Network registry identifying those with mucocutaneous Behçet’s disease (BD) may progres to major organ involvement (MOI)m especially at later stages.
Read ArticleA New Disease Activity Score for Antiphospholipid Syndrome?
APS is a systemic autoimmune disorder. It is characterised by the presence of antiphospholipid antibodies and a broad spectrum of clinical features, causing obstetric as well as thrombotic, microvascular and non-thrombotic (TMN) symptoms.
Read ArticleAPEX Study: Inhibition of structural damage progression with Guselkumab in Psoriatic Arthritis
Guselkumab, a human IL-23 inhibitor, was evaluated in patients with active psoriatic arthritis (PsA) and shown to have both clinical and radiographic benefits at weeks 24 and 48.
Read ArticlePulse Steroids and Mycophenolate in Juvenile Dermatomyositis
JAMA Dermatology has published a pilot study demonstrating the safety and efficacy of intermittent intravenous methylprednisolone pulse (IVMP) therapy plus mycophenolate in 28 patients with juvenile dermatomyositis (JDM).
Read ArticleSeeking Musculoskeletal Care
A report entitled The Burden of Musculoskeletal Diseases in the United States (BMUS) report was published by the US Bone and Joint Decade (USBJD) with input from rheumatology, orthopedic surgery, physical medicine and rehabilitation regarding musculoskeletal care.
Read ArticleRheumatology Supply and Demand (2009 - 2024)
A retrospective review of US rheumatology manpower and training between 2009 and 2024 shows that US rheumatology training has become competitive with an increasing surplus of applicants compared to available training positions, which have also increased over time.
Read Article


Links:


Dr. John Cush RheumNow ( View Tweet)
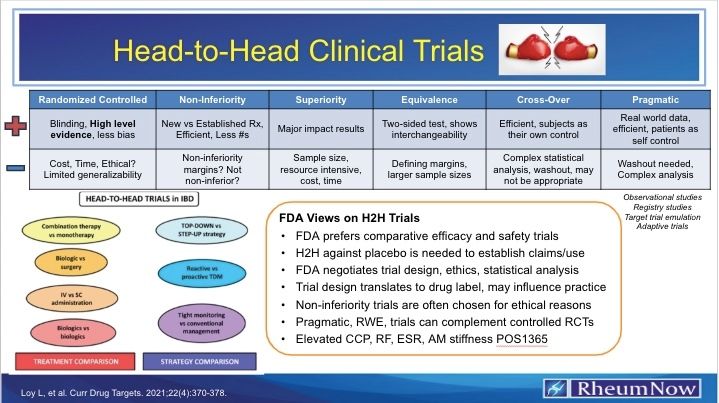


Dr. John Cush RheumNow ( View Tweet)