All News
Featured Abstracts from Industry at EULAR 2021
Many great presentations this week at EULAR 2021 will feature industry sponsored clinical trials and reports. Often these become the pivotal studies for regulatory approval and the annual congresses are their first look.
Read ArticleFoot Pain Commonly Overlooked in RA
Foot pain is a common but underappreciated symptom of rheumatoid arthritis (RA) and can be associated with worse disease, European researchers found.
Read ArticleRheumNow Podcast – Heart, Lung & Liver (5.28.2021)
Dr. Jack Cush reviews the news and journal reports from this past week on RheumNow.com.
Read ArticleMethotrexate Impairs COVID Vax Response
One-third of patients with immune-mediated inflammatory diseases being treated with methotrexate showed attenuated responses to the COVID-19 vaccine, researchers reported.
Read ArticleEfficacy of 6 Weeks Antibiotics with Prosthetic Joint Infections
The NEJM has published the results of a French multicenter study showing that prosthetic joint infections can be successfully managed with 6 weeks of antibiotic therapy; this was noninferior and had better outcomes compared to 12 weeks of antibiotic therapy.
Read Article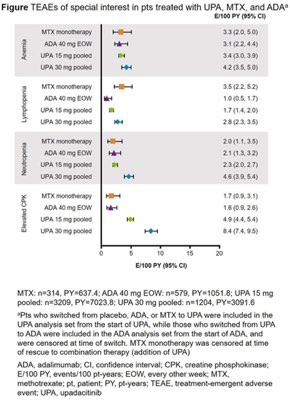

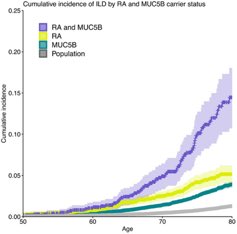
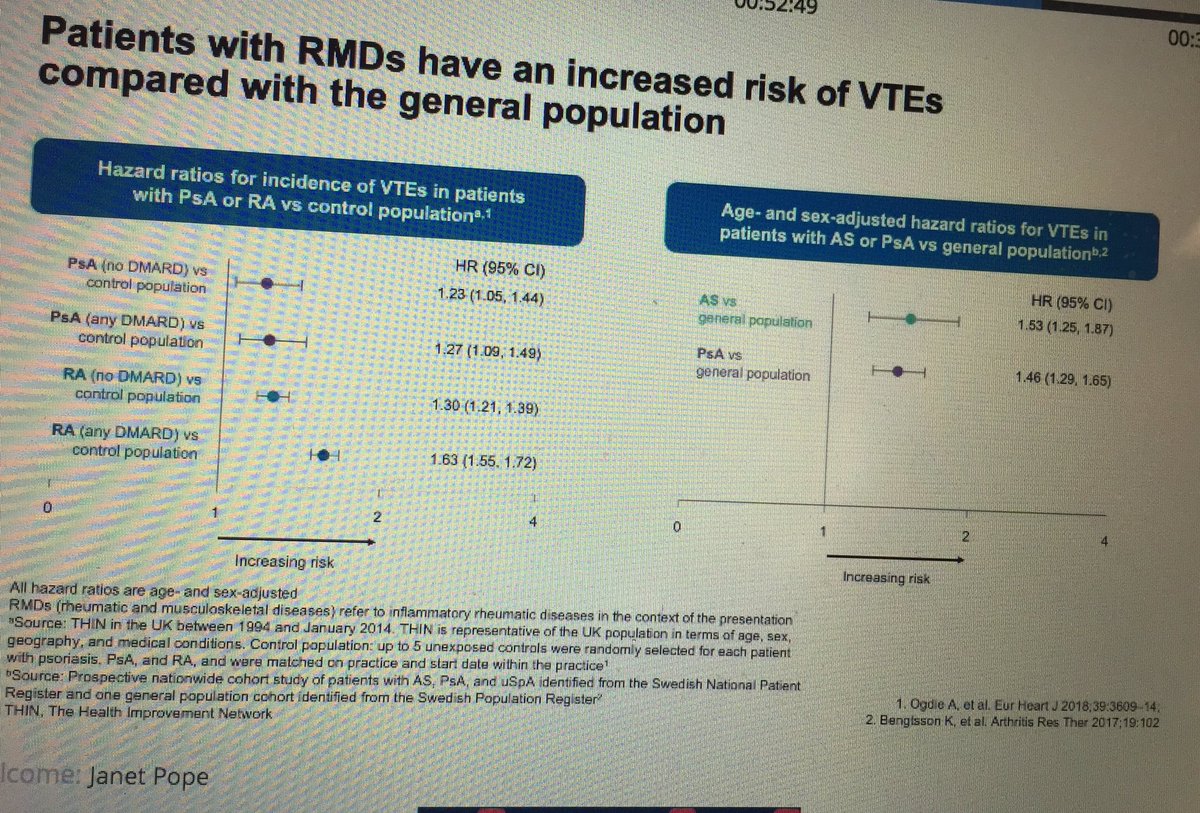
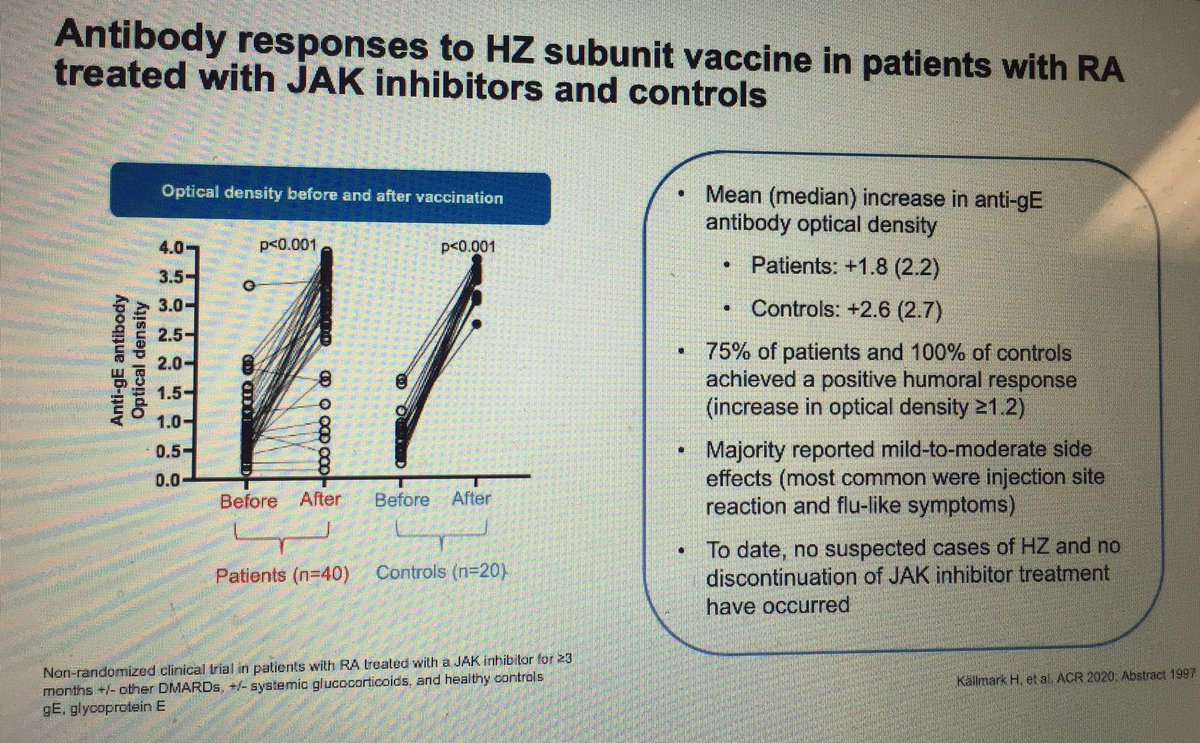
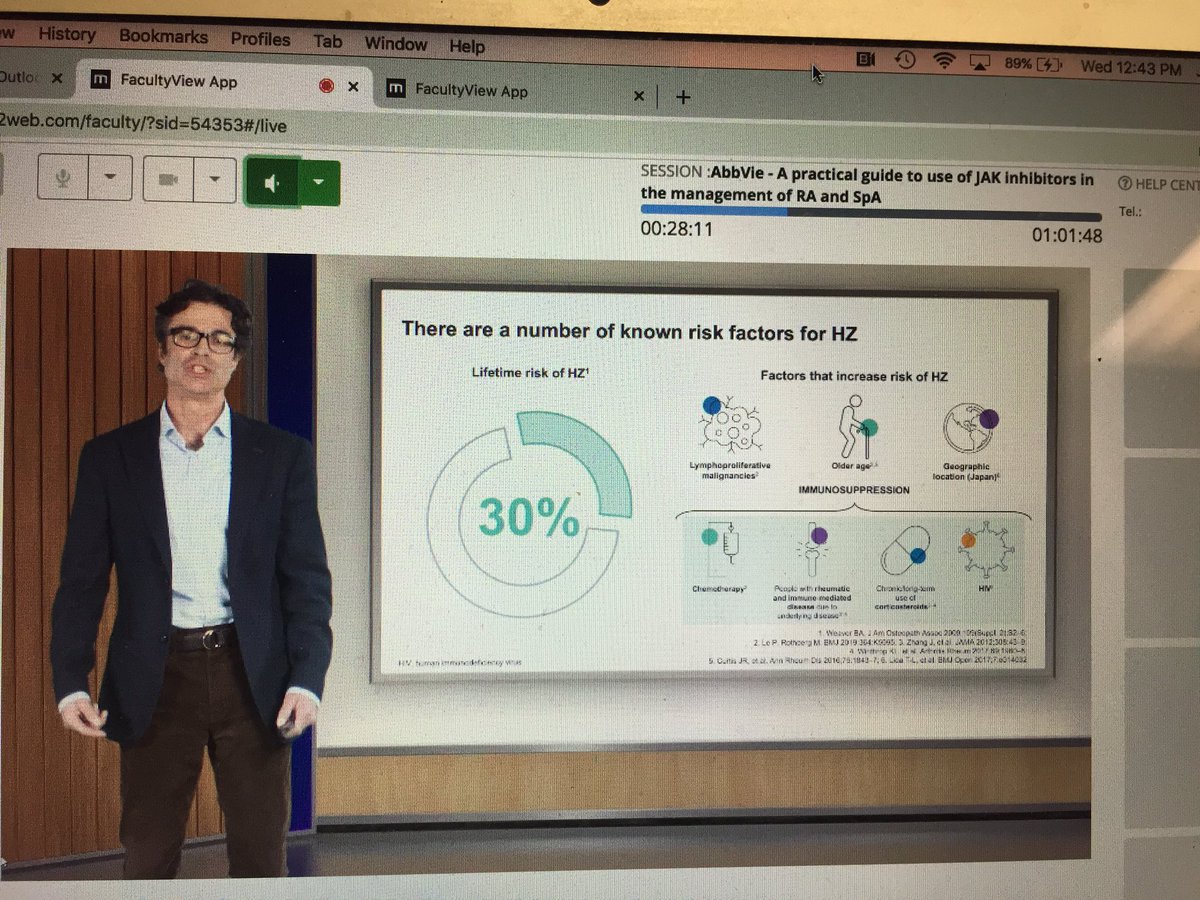
Janet Pope Janetbirdope ( View Tweet)


Links:
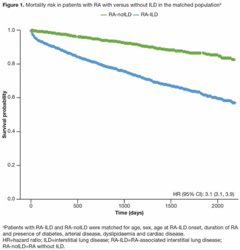
Dr. John Cush RheumNow ( View Tweet)

Links:
Links:

Links:
Dr. John Cush RheumNow ( View Tweet)

Links:

Links:
Dr. John Cush RheumNow ( View Tweet)
Links: