All News
EMA Final Update on JAK Inhibitors and MACE, Malignancy & VTE Risks
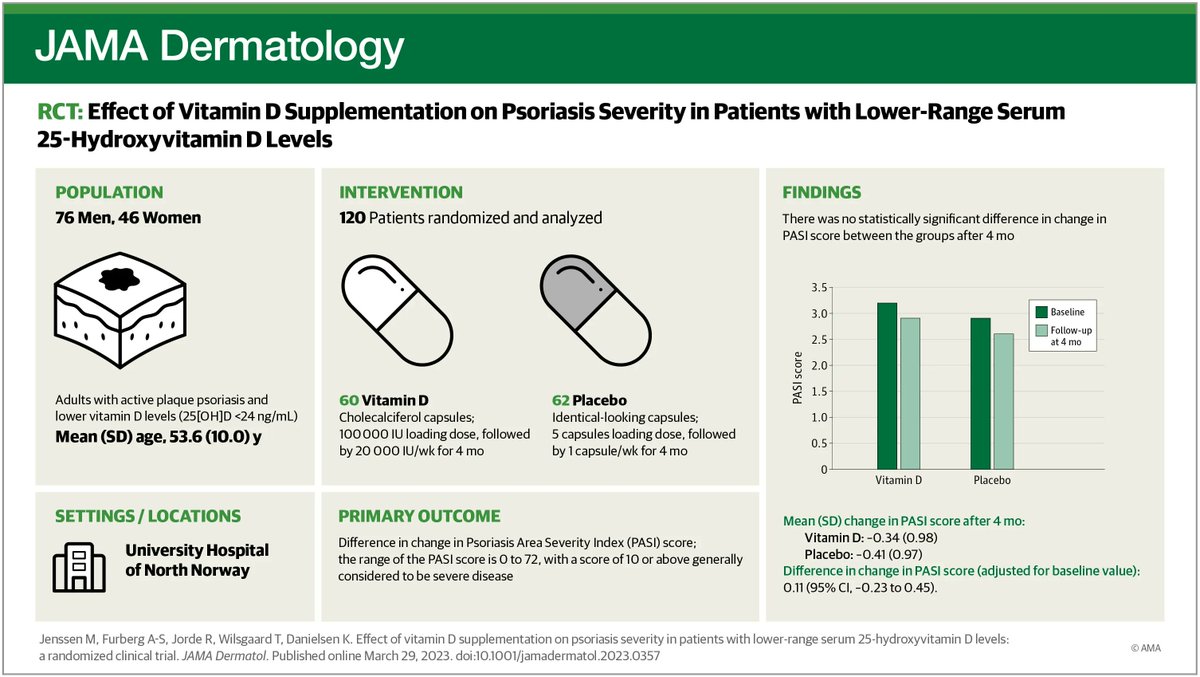
The EMA has updated recommendations regarding the use of JAK inhibitors by issuing a direct healthcare professional communication summarizing the data and warnings regarding an increased risk of malignancy, major adverse cardiovascular events (MACE), serious infections, VTE, and mortality in some patients receiving JAKi for the treatment of chronic inflammatory disorders.
Read ArticleThe Origin Story of RheumNow’s Campaign: Women in Rheumatology--The XX Factor
Origin stories have captivated me ever since my childhood spent reading Greek Mythology and Marvel comic books. Superheroes have different powers and personalities.
Read ArticleIntrathecal Methotrexate for Cerebritis (3.31.2023)
Dr. Jack Cush reviews the CV advantage to walking, the OA risk with running and the eczema-OA connection; these articles and journal reports from the past week on RheumNow.com.
Read Article
Links:
Dr. John Cush RheumNow ( View Tweet)

Links:

Links:
Dr. John Cush RheumNow ( View Tweet)
LAVLI - A New Autoinflammatory Disorder NIH researchers have have described a novel autoinflammatory disorder called "Lyn kinase-associated vasculopathy and liver fibrosis" (LAVLI), based on a mutation in the LYN gene. https://t.co/RYmb3QFB1T https://t.co/sn5hwVxwPk
Links:

Links:
Links:

Links:
Links:

Links:

Links:
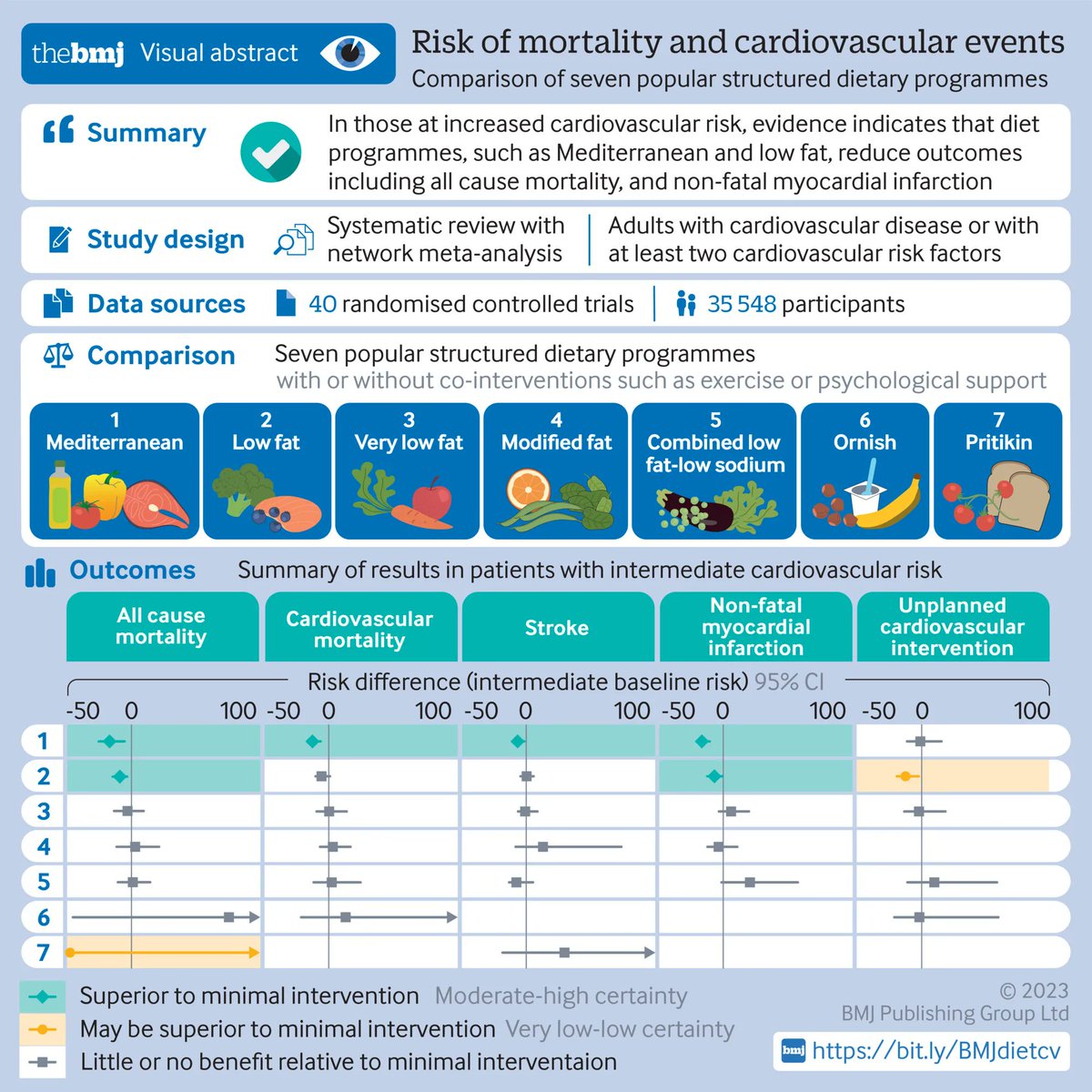
Links:

Links:

Links:
Links:

Links:

Links:






