FDA Outlines its Guidance for Biosimilars Save
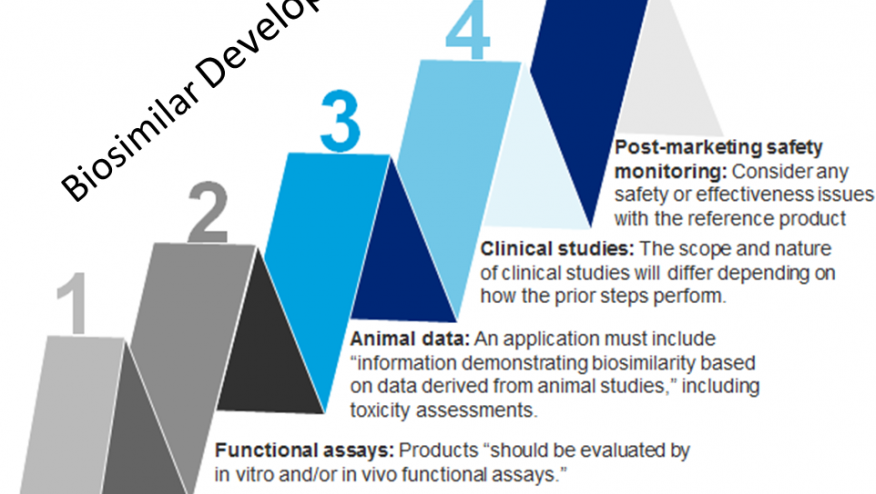
The FDA has released its guidance document on biosimilar development, stating that this represents its current thinking and may not be the final document. The document was published last week and will be instrumental in the development of biosimilar products in this country. In the guidance, the FDA outlines a stepwise process for demonstrating biosimilarity. The FDA guidance document can be found at the FDA website. http://www.fda.gov/downloads/










If you are a health practitioner, you may Login/Register to comment.
Due to the nature of these comment forums, only health practitioners are allowed to comment at this time.