All News
ACR Responds to CY2020 Medicare Physician Fee Schedule Proposed Rule
Rheumatology leaders commend CMS for proposing E/M code changes and urge agency to make additional changes to final rule.d
Read ArticlePersistent Inflammatory Arthritis After Immune Checkpoint Inhibitors
Braaten and colleagues from Johns Hopkins School of Medicine have reported their experience with chronic inflammatory arthritis induced by immune checkpoint inhibitor therapies, showing that in some, inflammatory arthritis persists after the immunotherapy has been discontinued.
Parenteral Out-Performs Oral Weekly Methotrexate
A systematic review in PLOS suggests that parenteral MTX therapy is more successful than oral MTX in achieving optimal disease activity control.
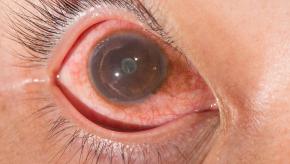
Read ArticleFDA Grants Breakthrough Status for Potential Lupus Nephritis Drug
Obinutuzumab (Gazyva) has been granted Breakthrough Therapy Designation (BTD) by the U.S. Food and Drug Administration (FDA) for use in adults with lupus nephritis (LN). The drug made by Genentech, is going forward based on the Phase II NOBILITY study in adult patients with proliferative lupus nephritis (LN). Currently, there are no FDA-approved medicines for lupus nephritis.
Read ArticleACR Survey Shows Half of Patients Cannot Afford Treatments
Americans living with rheumatic disease face significant healthcare challenges, according to a national patient survey released this week by the American College of Rheumatology. More than 1,500 U.S. adults living with rheumatic disease responded to the survey, which asked a range of questions related to healthcare access, affordability and lifestyle. Key findings include that even though 90 percent of respondents reported having health insurance coverage, nearly 60 percent said they had difficulty affording their medications or treatments in the past year.
Read ArticleMedical Use of Cannabis in 2019
JAMA has published an overview of cannabis and its medical uses. Although nearly 10% of cannabis users in the United States report using it for medicinal purposes, there is insufficient evidence to support the use of medical cannabis for most conditions for which its use is advocated or advised. Nevertheless, there is increase in favoring the public availability of cannabis, largely for the management of more than 50 medical conditions.
Read ArticlePrior Authorizations Delay Care in Rheumatology
Physicians who believe their patients' health is negatively affected by insurers' demands for prior authorization, and the delays that often result, will find that opinion vindicated by a new study of rheumatology care: when permission had to be sought from insurers to provide intravenous drugs,
Read ArticleBimekizumab Add-on Therapy in Rheumatoid Arthritis
Bimekizumab is a dual inhibitor of IL-17A and IL-17F that has been shown to be effective in psoriasis and psoriatic arthritis. A proof-of-concept study shows that giving bimekizumab to rheumatoid arthritis patients not adequately controlled by certolizumab pegol resulted in a rapid decrease in disease activity achieved after 12 weeks of treatment. These findings are novel as anti-IL-17 monoclonal antibody therapy has previously been shown to be ineffective in RA.
Read Article74 Percent of Rheumatoid Arthritis Patients Dissatisfied with Treatment
CreakyJoints has completed a 258 patient survey showing that nearly three-fourths of people with rheumatoid arthritis (RA) have expressed dissatisfaction with their treatments, including conventional (csDMARDs) and biologic Disease Modifying Antirheumatic Drugs (bDMARDs).
Read ArticleSteroid Sparing Effects of Methotrexate and Mycophenolate in Uveitis
Patients with noninfectious uveitis (intermediate, posterior uveitis, or panuveitis) often require high dose corticosteroids and therefore may need steroid-sparing DMARD therapy. The FAST study investigated the corticosteroid-sparing effect of methotrexate or mycophenolate mofetil in adults with noninfectious uveitis.
Read ArticleAnti-IL-23 Beats IL-17 in Plaque Psoriasis
Lancet reports a head-to-head trial of antibodies against interleukin (IL)-23 and IL-17A in patients with moderate-to-severe psoriasis favored guselkumab with superior PASI 90 responses at week 48 (compared to secukinumab).
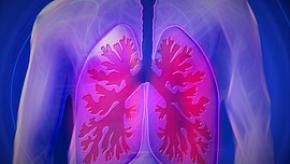
Read ArticleNintedanib FDA Approved for Scleroderma Lung Disease
Last Friday, the US Food and Drug Administration approved Ofev (nintedanib) to slow the rate of decline in pulmonary function in adults with interstitial lung disease associated with systemic sclerosis or scleroderma, called SSc-ILD.
ILD as a complication of SSc may lead to progressive loss of lung function and may be associated with a significant mortality risk. Prior to the approval of Olev, there were no FDA approved drugs for SSc-ILD.
RA Women are Less Likely to Breastfeed
A large pregnancy registry has published their results showing rheumatoid arthritis (RA) patients who become pregnant are less likely to breastfeed compared to non-RA women from the general population, with many women stopping breastfeeding so that they could start medication, even though many of
Read ArticleRespiratory Risks Not Increased in RA Patients with COPD
An insurance claims based study of RA patients with COPD shows that biologics do not have an increased rate of respiratory events compared to those on conventional DMARDs.
A real world cohort of RA patients with COPD was drawn from US-based MarketScan databases. Patients on biologic DMARDs and/or targeted synthetic DMARDs (tsDMARDs) were propensity matched to those on conventional synthetic DMARDs (csDMARDs).
Tocilizumab Shows No Increase in Cardiovascular Risk
The ENTRACTE trial examined the risk for major adverse cardiovascular events (MACE) in RA patients and found no increased risk of MACE in patients treated with tocilizumab (TCZ) versus etanercept (ETN).
Read ArticleRheumNow Podcast - I Wanna New Drug (8-30-19)
Dr. Jack Cush vents on choosing new therapies in rheumatoid arthritis.
Read Article
High-Dose Vitamin D: No Help for Bone Health
Vitamin D might not be much help for strengthening bones among healthy adults without osteoporosis, Canadian researchers reported, even at doses far higher than recommended daily allowances.
In a clinical trial assessing three levels of daily vitamin D supplementation -- 400 IU, 4,000 IU, and 10,000 IU -- radial volumetric bone mineral density was significantly lower among those (ages 55-70) taking higher doses for 3 years, according to Steven Boyd, PhD, of the University of Calgary in Canada, and colleagues.
Taltz FDA Approved for Ankylosing Spondylitis (Radiographic Axial SpA)
The FDA has approved the IL-17A inhibitor Taltz (ixekizumab) for the treatment of adults with active ankylosing spondylitis (AS: also known as radiographic axial spondyloarthritis).
Read ArticleACR/SPARTAN Recommendations for the Treatment of Ankylosing Spondylitis and Nonradiographic Axial Spondyloarthritis
The American College of Rheumatology (ACR), in partnership with the Spondylitis Association of America (SAA) and the Spondyloarthritis Research and Treatment Network (SPARTAN), released the 2019 Update of the Recommendations for the Treatment of Ankylosing Spondylitis (AS) and Nonradiographic Axi
Read ArticleRheumNow Podcast – Upadacitinib FDA Approved for RA (8.23.19)
Dr. Jack Cush reports on the news and journal reports from the past week on RheumNow.com, including: how your genetics may shape your microbiome; GERD as a risk factor for TMJ?; how can MDA-5+ dermatomyositis be any worse; new drug happenings; StillsNow.com; and more.
Read Article