All News
RheumNow Podcast - Despite Corona (3.6.20)
Dr. Jack Cush reviews the news and journal articles from the past week on RheumNow.com.
Need for Disruptive Innovation in Rheumatology
A full-read, novel Viewpoint article published in the Annals of Rheumatic Disease (Huizinga TWJ, et al) spotlights a recent international meeting of big thinkers, scientific collaborators and industry dedicated to innovation in rheumatology.
Read ArticleVenom Peptide-Steroid Conjugate Effective in Collagen Induced Arthritis
Science Translational Medicine features a report from the Fred Hutchinson Cancer Research Center showing that a scopion venom peptide, coupled to steroid, can significantly reduce joint inflammation in a rat model of rheumatoid arthritis (RA).
Read ArticleUpdates on Psoriatic Arthritis at ACR 2018
I think 2018 was the year that belonged to psoriatic arthritis (although some may argue in favor of immune conditions induced by Checkpoint inhibitors). Many years ago the late Professor Verna Wright in Leeds (UK) was a lone voice for many years describing the clinical subtypes of Psoriatic arthritis as something distinctive from rheumatoid arthritis. For a long time we thought that the treatments for PsA were just the same as RA since we were treating the same problem of synovitis.
Read ArticleDrug Interactions with Cannabinoids
Cannbinoids are widely available and used for a variety of indications, but little is known about their safety and their potential for drug interactions. The Canadian Medical Journal has published a review of drug interactions with cannabinoids, many of which are mediated by cytochrome-P450 metabolism.
Read ArticleAirway Inflammation Drives Rheumatoid Risk
A cohort analysis of the Nurses' Health Study suggests that asthma and COPD are associated with increased risk for incident rheumatoid arthritis (RA), independent of smoking- thus airway inflammation may be an important factor in the evolution of pre-cllnical RA.
Read Article
Links:
Dr. John Cush RheumNow ( View Tweet)

Dr. John Cush RheumNow ( View Tweet)

Dr. John Cush RheumNow ( View Tweet)

Links:
Dr. John Cush RheumNow ( View Tweet)

Dr. John Cush RheumNow ( View Tweet)

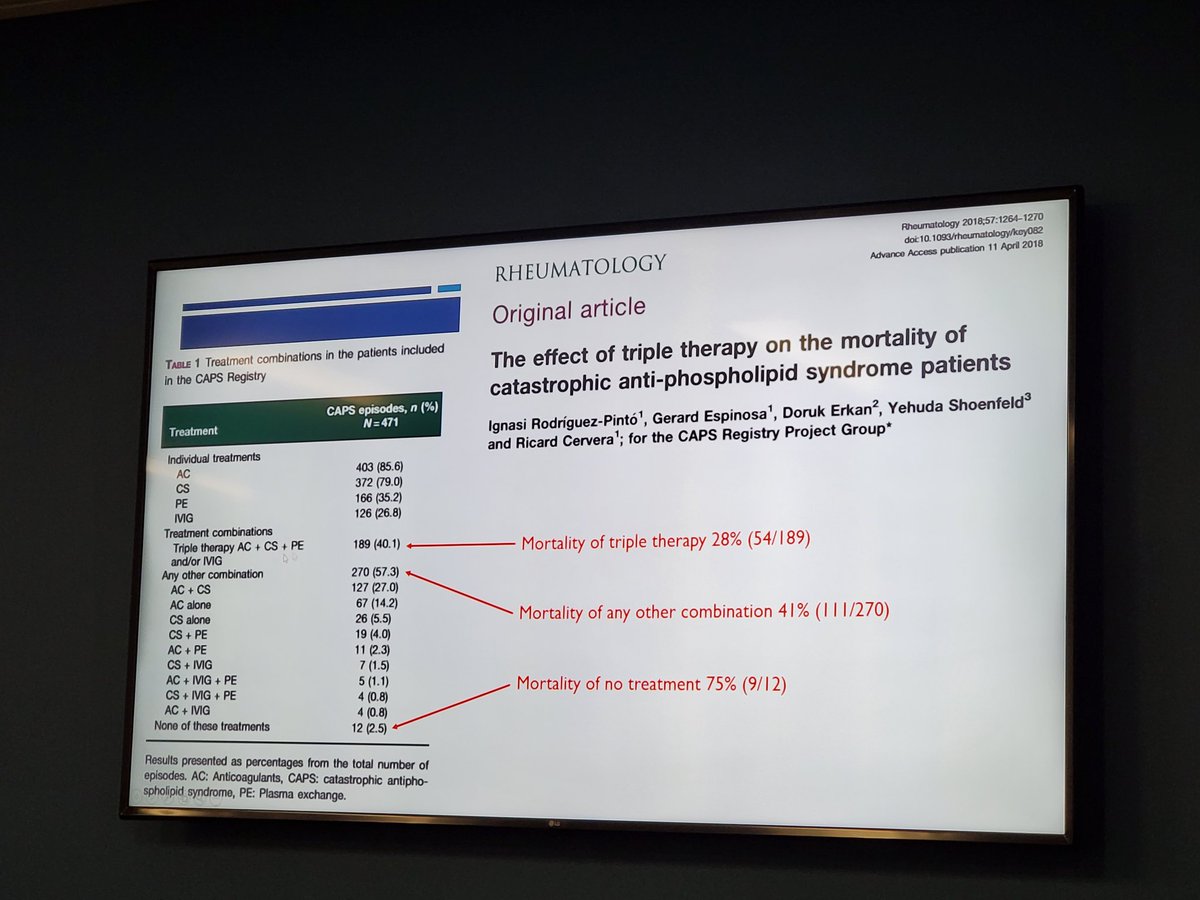
CAPS, best outcomes with triple therapy that includes Apheresis. Dr Compton #Utsw Rheum Grand Rounds https://t.co/MVesTmSNQK

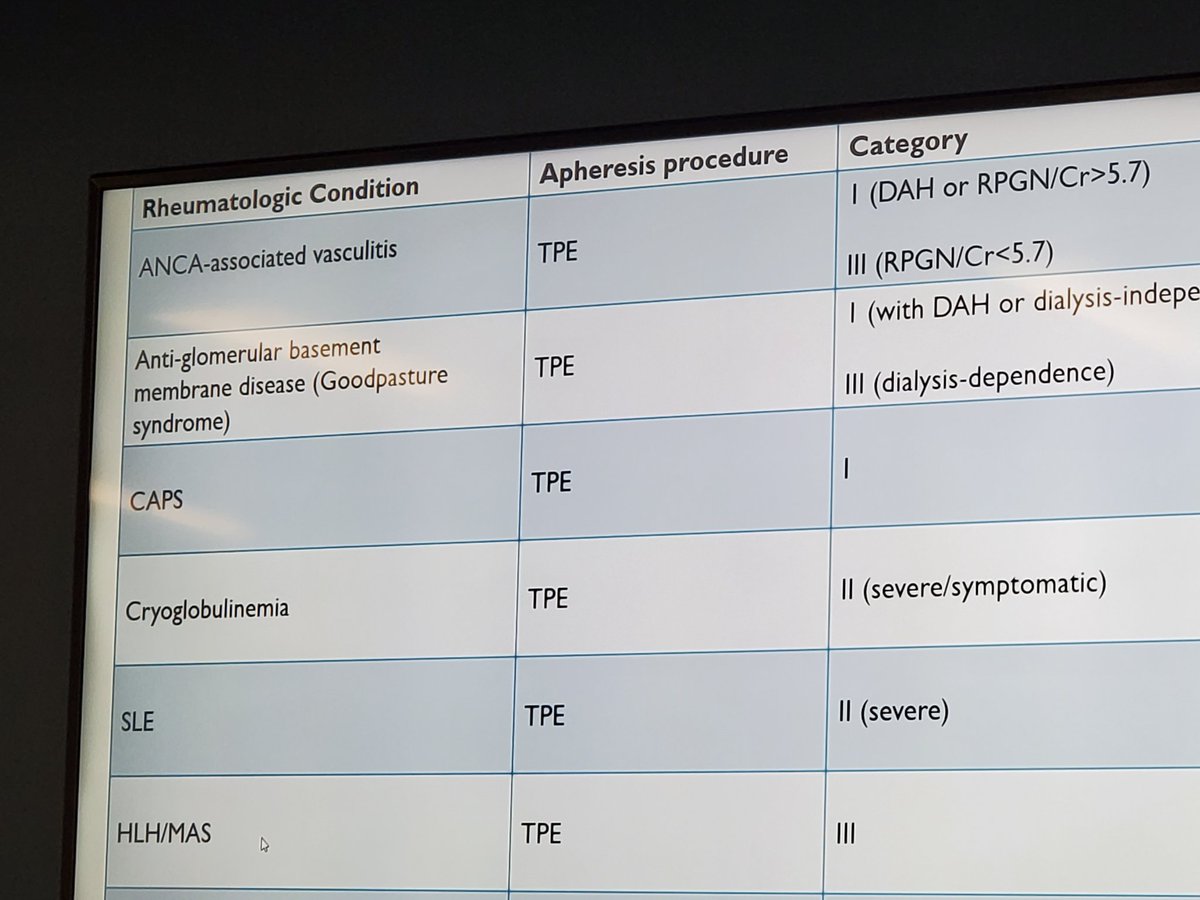
Criteria for CAPS. Dr. Compton #Utsw Rheum Grand Rounds https://t.co/tdVWhMEII3
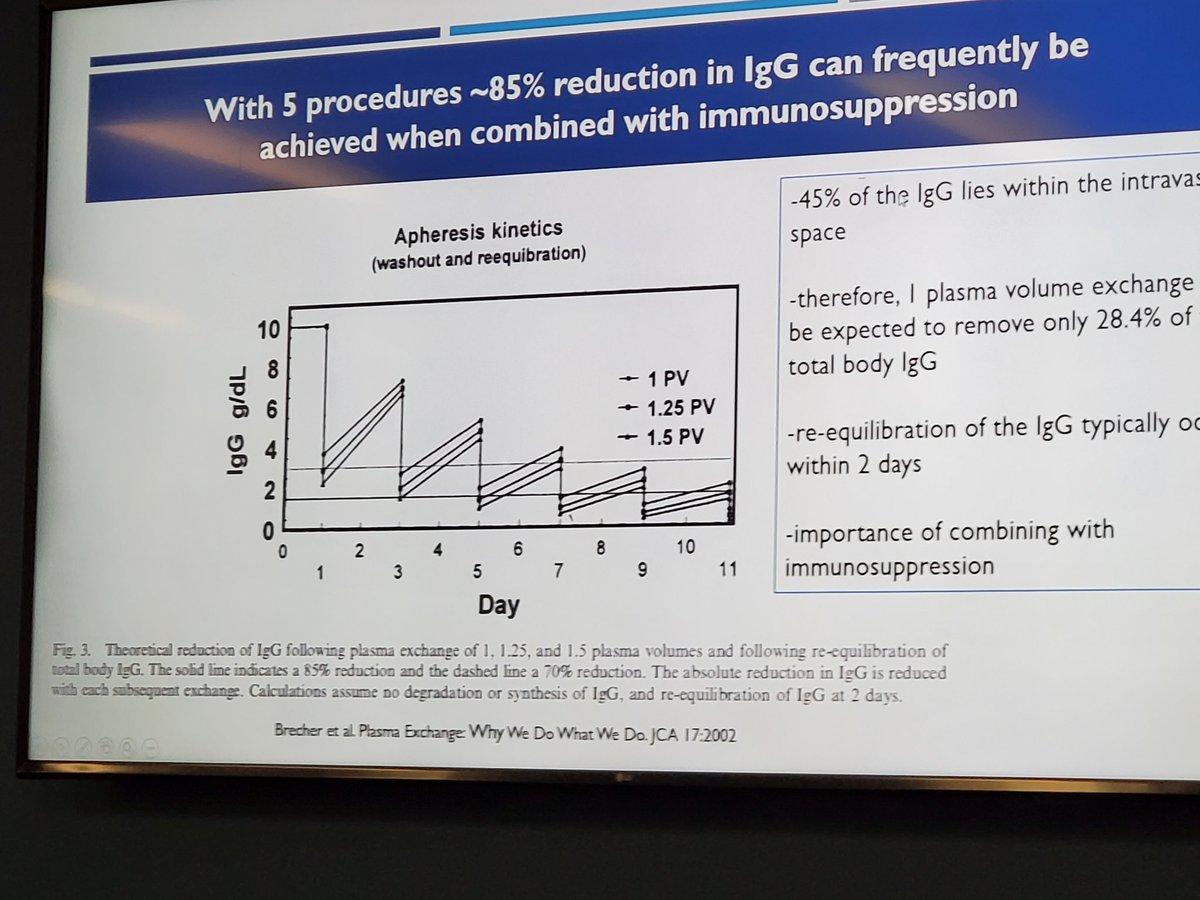


Dr. John Cush RheumNow ( View Tweet)

Dr. John Cush RheumNow ( View Tweet)

Links:
Dr. John Cush RheumNow ( View Tweet)