All News
Novel Gel Drug Delivery Developed for Rheumatoid Arthritis
Scientists at the Institute for Basic Science have invented a hydrogel capable of delivering drug at sites of inflammation in disorders such as rheumatoid arthritis.
Read ArticleBSR Guidelines on Lupus Management
In the UK, NICE has accredited the British Society of Rheumatology (BSR) to develop a guidance document on the management of systemic lupus erythematosus in adults. The last published guidelines for lupus were published in 2008 by EULAR and in 2012 by the ACR.
Read Article2016 EULAR Guidelines on RA Management
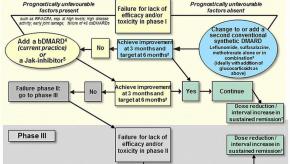
The management of rheumatoid arthritis (RA) has evolved significantly with time. Nevertheless, there are still some uncertainties - such as when, what and which biologic or novel therapy should be used.
Read ArticleThe RheumNow Week in Review - 6 October 2017
The RheumNow Week in Review discusses the past week's news, journal articles and highlights from RheumNow.com. In this week's report, Dr. Jack Cush discusses when to hold the biologic, lymphoma risk with tofacitinib, early clues to the diagnosis of RA, biologic use in pregnancy, what's killing psoriasis patients and the 2016 top 5, best selling drugs in rheumatology.
Read ArticlePatterns of Biologic Use During Autoimmune Pregnancy
While it is highly desirable to avoid medications during conception and pregnancy, statistics show that >90% of women take at least one drug during pregnancy and nearly half will take 3 or more medications during pregnancy.
Read ArticleAmgen-Abbvie Settle Humira Biosimilar Patent Dispute
Amgen's biosimilar version of adalimumab (Humira) was FDA-approved in September 2016 and given the trade name Amjevita (generic: adalimumab-atto). This new TNF inhibitor biosimilar has not yet been to market because of legal wranglings over patent issues by Abbvie's Humira.
Read Article29 September 2017 The RheumNow Week in Review
The RheumNow Week in Review discusses the past week's news, journal articles and highlights from RheumNow.com. This week's report discusses regulatory actions by NICE and FDA, higher death rates in RA and psoriasis, increased risk of RA with Asthma, rising numbers for OA, RA, and STDs.
Read ArticlePsoriasis Increases Risk of Major Adverse Cardiovascular Events
A report in the Journal of the American Academy of Dermatology shows that psoriasis duration and inflammation may result in cardiovascular inflammation and an increased risk of major adverse CV events.
Read ArticleIncreased Deaths in RA, Despite Decreasing Mortality Rates
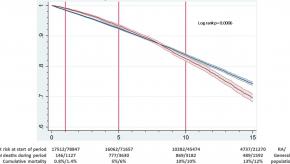
Data from the Swedish Rheumatology Quality (SRQ) Register studied death rates in patients with rheumatoid arthritis (RA) between 1997 and 2014. Holmqvist and colleagues studied 17,512 RA and 78,847 matched controls from the general population, until their death.
Read ArticleNo Cancer Risk With Biologic Use
A polulation-based study from Sweden has shown that treatment with tocilizumab, abatacept, rituximab, or tumor necrosis factor (TNFi) inhibitors does not affect the risk of malignant neoplasms among patients with rheumatoid arthritis. Specifically, use of a first or second TNFi or biologic DMARDs (bDMARD) does confer a different cancer risk when compared to conventional DMARDS in biologic–naive RA patients.
Read ArticleFDA Panel Backs New Zoster Subunit Vaccine
GlaxoSmithKline has announced that the Vaccines and Related Biological Products Advisory Committee (VRBPAC) of the US Food and Drug Administration (FDA) voted unanimously in support of the efficacy, safety and approval of its herpes zoster subunit (HZ/su) vaccine (called Shingrix) for the prevention of herpes zoster (shingles) in adults ages 50 and over.
Read ArticleSirukumab Turned Down by FDA
On Friday the U.S. Food and Drug Administration announced that will not approve Johnson & Johnson’s rheumatoid arthritis drug sirukumab, stating further data and study would be needed to establish its safety.
Read ArticleRomosuzumab Followed by Alendronate is Best in Fracture Prevention
The NEJM reports that in high risk post-menopausal women, romosozumab for 12 months followed by alendronate resulted in a significantly lower fracture than alendronate alone.
Read ArticleCDC: 40% of U.S Adults Claim to Have Arthritis
The CDC has reported its 2013 and 2014 prevalence statistics for arthritis and other chronic medical conditions affecting U.S. adults aged ≥18 years. Data is drawn from the ongoing Behavioral Risk Factor Surveillance System (BRFSS), a state-based, telephone survey of noninstitutionalized adults. Data herein is self-reported arthritis (OA, RA, Gout, FM) and is quantified by state and metropolitan areas.
Read Article15 September 2017 The RheumNow Week in Review
The RheumNow Week in Review discusses the past week's news, journal articles and highlights from RheumNow.com. This week's report discusses metabolic syndrome in lupus, bisphosphonate holidays, vasculitis and vascular inflammation, vaccination, and the repeated wonders of Vitamin D.
Read ArticleNew Zoster Vaccine Recommended by FDA Panel
Reuters reports that the FDA advisory panel has voted 11-0 in favor of the safety and efficacy and ultimate approval of GlaxoSmithKline’s Shingrix shingles vaccine for use in adults aged 50 and over.
Read ArticleNew Recommendations on Biosimilar Use
The introduction of a growing number of biosimilars into the market poses a substantial change in cost of care for patients with inflammatory rheumatologic disorders.
Read ArticleIncreasing Deaths and Breaking Bad with Fentanyl
Opioid overdose deaths quadrupled from 1999 to 2015 and accounted for 63% of drug overdose deaths in the United States in 2015. During 2010–2015, heroin overdose deaths quadrupled from 3,036 to 12,989, with heroin and illicitly manufactured fentanyl (IMF) as likely contributors to this trend.
Read ArticleDSB Reports & Updates – August 2017
This Drug Safety Bulletin address FDA delay of baricitinib, etanercept effective at room temperature, Consumer Reports features on drug safety, no association between Alzheimer's and PPIs, AHRQ review of opioid drug safety, FDA safety abeling changes, drug shortages and more.
Read ArticleCanakinumab Patients have Lower Risk of Lung Cancer
The CANTOS trial has shown that interleukin 1β inhibition by Canakinumab (CAN) resulted not only in a reduction of cardiovascular deaths but also significantly decreased the incidence and death from lung cancer.
Read Article