2016 EULAR Guidelines on RA Management Save
The management of rheumatoid arthritis (RA) has evolved significantly with time. Nevertheless, there are still some uncertainties - such as when, what and which biologic or novel therapy should be used.
A large international Task Force was established by EULAR to develop evidence based decisions from 3 systematic literature reviews. As such this panel has published 4 overarching principles and 12 recommendations. These recommendations address the use of conventional synthetic (cs) disease-modifying antirheumatic drugs (DMARDs) as well as biologic DMARDs (bDMARDs). These guidelines are updated from that published in 2013.
Overarching Principles
- Treatment of patients with RA should aim at the best care and must be based on a shared decision between the patient and the rheumatologist.
- Treatment decisions are based on disease activity and other patient factors, such as progression of structural damage, comorbidities and safety issues.
- Rheumatologists are the specialists who should primarily care for patients with RA.
- RA incurs high individual, medical and societal costs, all of which should be considered in its management by the treating rheumatologist.
EULAR recommendations
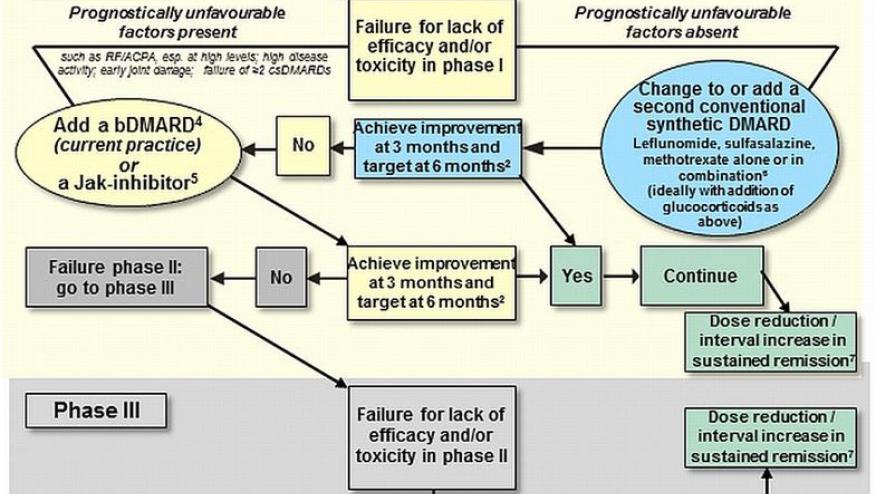
- Therapy with DMARDs should be started as soon as the diagnosis of RA is made
- Treatment should be aimed at reaching a target of sustained remission or low disease activity in every patient.
- Monitoring should be frequent in active disease (every 1–3 months); if there is no improvement by at most 3 months after the start of treatment or the target has not been reached by 6 months, therapy should be adjusted.
- MTX should be part of the first treatment strategy.
- In patients with a contraindication to MTX (or early intolerance), leflunomide or sulfasalazine should be considered as part of the (first) treatment strategy.
- Short-term GC should be considered when initiating or changing csDMARDs, in different dose regimens and routes of administration, but should be tapered as rapidly as clinically feasible
- If the treatment target is not achieved with the first csDMARD strategy, in the absence of poor prognostic factors, other csDMARDs should be considered.
- If the treatment target is not achieved with the first csDMARD strategy, when poor prognostic factors are present, addition of a bDMARD* or a tsDMARD* should be considered; current practice would be to start a bDMARD
- bDMARDs and tsDMARDs should be combined with a csDMARD; in patients who cannot use csDMARDs as comedication, IL-6 pathway inhibitors and tsDMARDs may have some advantages compared with other bDMARDs
- If a bDMARD or tsDMARD has failed, treatment with another bDMARD or a tsDMARD should be considered; if one TNF-inhibitor therapy has failed, patients may receive another TNF-inhibitor or an agent with another mode of action
- If a patient is in persistent remission after having tapered GC, one can consider tapering bDMARDs, especially if this treatment is combined with a csDMARD.
- If a patient is in persistent remission, tapering the csDMARD could be considered.

Abbreviations: bDMARD, biological DMARD; bsDMARD, biosimilar DMARDs; csDMARDs, conventional synthetic DMARDs; DMARDs, disease-modifying antirheumatic drugs; EMA, European Medicines Agency; FDA, Food and Drug Administration; IL, interleukin; MTX, methotrexate; RF, rheumatoid factor; TNF, tumour necrosis factor; tsDMARDs, targeted synthetic DMARDs.










If you are a health practitioner, you may Login/Register to comment.
Due to the nature of these comment forums, only health practitioners are allowed to comment at this time.