All News
No Cancer Recurrence with Biologic DMARDs in RA
A prospective Danish rheumatoid arthritis (RA) registry study look at the risk of recurrence when a biological disease-modifying antirheumatic drugs (bDMARDs) was ued in patients with a a prior solid cancer (in remission) and found no increased cancer
Tocilizumab vs. Methotrexate in Rheumatoid Arthritis
A large randomized rheumatoid arthritis (RA) clinical trial compared subcutaneous tocilizumab (TCZ) vs oral methotrexate (MTX) vs. the combination of subcutaneous TCZ and MTX, and showed that TCZ was superior to MTX, either given as TCZ monotherapy or in combination with MTX.
Read ArticleMoving Targets in Lupus and Lupus Trials
Clinical trial data quality is a moving target. Lupus is also a moving target. Can we address the moving target of lupus pathology with the moving targets of quality clinical trials and scientific treatment selection?
Read ArticleIs Lupus Just a B Cell Disorder?
I'm very happy to give you some insights entitled, “Is Lupus a B-Cell Disorder”. I will review some aspects, mainly pros and cons, but at the end you may forgive me, I will escape a little bit from a clear yes or no.
Read ArticleSteroids in Lupus Nephritis: No Free Lunch
Today I'm here to talk with you about steroids in lupus nephritis. The impetus for this is the 2024 ACR guideline for the management of lupus nephritis.
Read ArticleBiologics in Pregnancy Patients With Autoimmune Disease
A large cohort, claims data study shows that among pregnant women receiving biologic therapies for autoimmune conditions, 72% continued their biologics pregnancy, more so among inflammatory bowel disease (IBD) patients than those with rheumatoid arthritis (RA), psoriasis (PsO) or psoriatic arthri
Read Article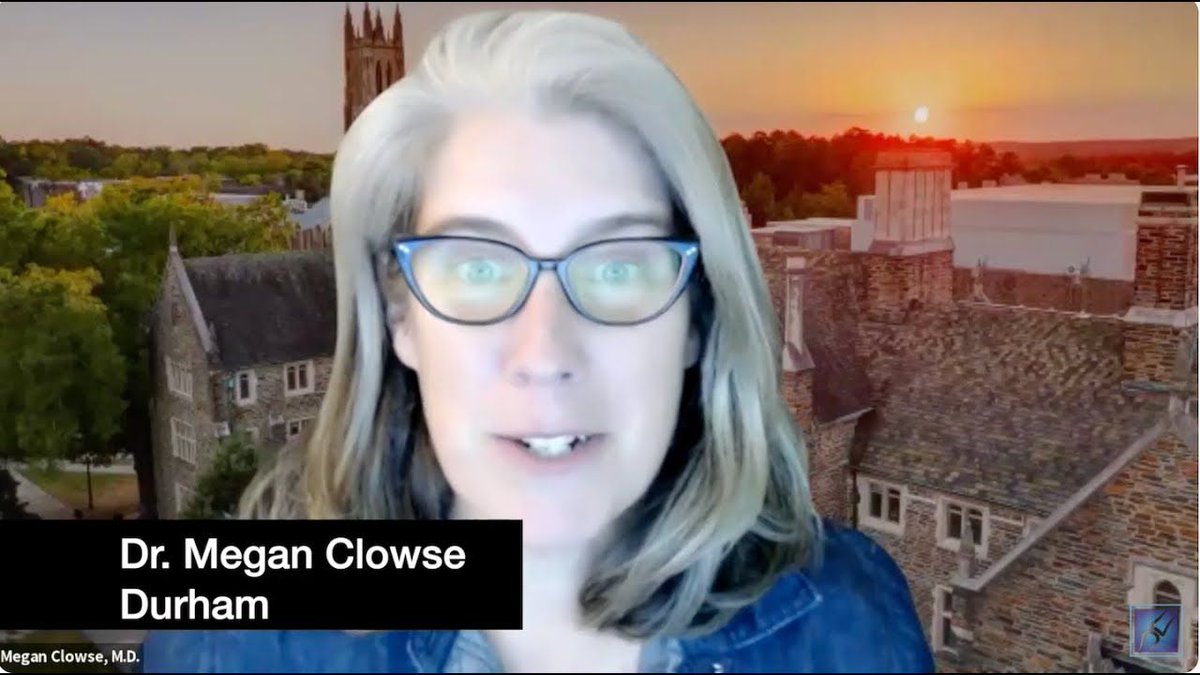








Comparing EULAR (2025) and ACR (2020) Guidelines on Safety of Lupus Medications in Pregnancy Attendees at the 2025 RheumaPreg meeting were excited to discuss the newly released EULAR recommendations for use of antirheumatic drugs in reproduction, pregnancy, and lactation. https://t.co/csd0FE08ZO

Links: