Biologics in Pregnancy Patients With Autoimmune Disease Save

A large cohort, claims data study shows that among pregnant women receiving biologic therapies for autoimmune conditions, 72% continued their biologics pregnancy, more so among inflammatory bowel disease (IBD) patients than those with rheumatoid arthritis (RA), psoriasis (PsO) or psoriatic arthritis (PsA).
Complex autoimmune disorders become more complex if the patient receives biologic therapy or if the patient becomes pregnant. In such instances, ongoing biologic use depends on patient activity, pregnancy related risks and the efficacy/safety profile of biologic therapy to bo the mother and fetus.
Merative MarketScan Research Database study identified 6131 pregnant patients with an autoimmune condition who used a biologic at least once during pregnancy. This included patients with Crohn disease (25.6%), RA (24.1%), ulcerative colitis, PsO, PsA, ankylosing spondylitis (AS), systemic lupus erythematosus (SLE), and multiple sclerosis (MS).
During the 10 year study period from 2011 to 2021, there was a gradual but significant increase in the use of biologics among pregnant autoimmune patients (from 64% in 2011 to 75.2% in 2021; P < .001). Among the biologics studied, TNF inhibitors were the most frequent biologic agents used during pregnancy (first trimester [80.5%]; second trimester [84.4%] and third trimester[83.5%]) and post partum [83.4%]. Other biologics (vedolizumab, abatacept, natalizumab, belimumab), also showed increasing use, from 2.3% in 2011 to 14.4% in 2021.
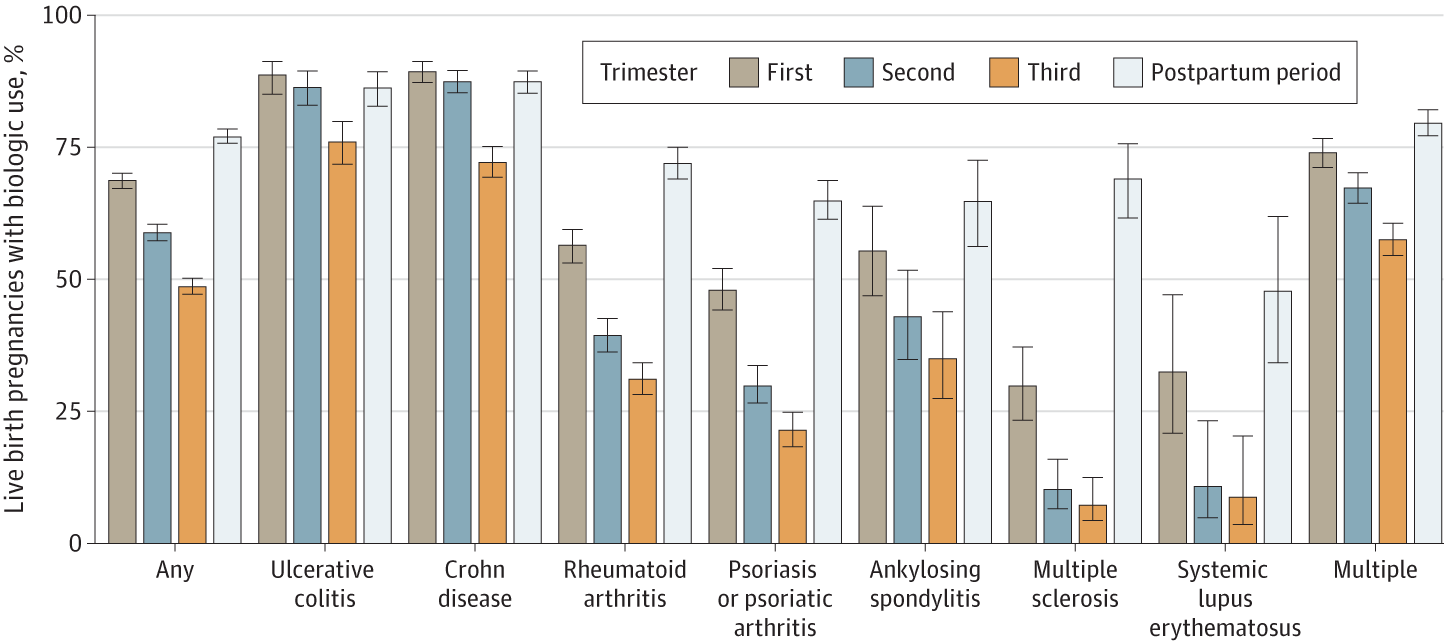
Among live birth pregnancies, biologic use declined throughout gestation:
- 2981 patients (68.6%) 1st trimester
- 2555 patients (58.8%) 2nd trimester
- 2113 patients (48.6%) 3rd trimester
- Postpartums 3350 patients (77.1%) were back on biologic therapy.
Compared to RA, Crohn disease (OR 7.88; CI, 5.93-10.47) and ulcerative colitis (OR, 5.35; 3.73-7.66) patients were more likely to remain on biologic therapy. Less likely to stay on biologics were pregnant women with PsO or PsA (OR, 0.65; 0.52-0.80), MS (OR 0.19) and SLE (OR 0.29).
Rules for biologic use during pregnancy vary by specialty and autoimmune diagnosis. Indication-specific risk-benefit assessments of biologic use are needed.










If you are a health practitioner, you may Login/Register to comment.
Due to the nature of these comment forums, only health practitioners are allowed to comment at this time.