All News
FDA Panel Votes 10-4 to Approve Lesinurad for the Treatment of Hyperuricemia/Gout
Despite concerns over safety, the FDA Arthritis Advisory Committee voted favorably to approve lesinurad (LES) at a meeting held Friday, October 23, 2015. Overall, panel members felt the potential benefits of LES outweighed the safety concerns reviewed by the FDA presenters.
Read ArticleIncreasing Placebo Responses in the USA
A recent analysis of randomized clincal trials (RCT) for the treatment of neuropathic pain has shown in increase in placebo responses. (Citation source http://buff.ly/1LL8yAi)
Read ArticleU.S. Pays 10 Times More for Prescription Drugs than Other Countries
Prescription drugs may cost up to 10 times more in the United States than they do in other countries, according to a 2013 Comparative Price Report was released last month by the International Federation of Health Plans (IFHP).
Read ArticleFDA Reviews the Safety of Lesinurad in Gout
Lesinurad is a novel URAT-1 inhibitor being developed as a urate-lowering therapy for gout by Astra-Zeneca.
Read ArticleFDA Holds Off on Xeljanz Approval for Psoriasis
The FDA has denied Pfizer's application seeking an indication for Xeljanz in patients with moderate to severe chronic plaque psoriasis.
Read ArticleICD-10 Headlines
Nearly 2 weeks have passed since the painful launch of ICD-10 in US medical practices.
Read ArticlePrescription Opioid Use Falls While Abuse Rises
From 2003 to 2013, the percentage of nonmedical use of prescription opioids decreased among adults in the U.S., while the prevalence of prescription opioid use disorders, frequency of use, and related deaths increased, according to a study in the October 13 issue of JAMA.
Read ArticleLupus Foundation of America Announces 2015 Award Recipients
Healio reported the newly announced grant recipients from the Lupus Foundation of America.
Read ArticleICD-10: Preparing for the Next Y2K
ICD-10 starts tomorrow, October 1st. Your Coding memory will have to increase 5-fold or more as we go from 16,000 to 68,000 new ICD-10 codes. How this will affect each of us remains to be seen. There’s a lot to learn, and many wonder if this will be the next “Y2K”.
Read ArticleDSB: Drug Shortages - September 2015
RheumNow is committed to reporting safety issues in our monthly Drug Safety Bulletins. Each month we will update you with reports of new, ongoing and resolved Drug Shortages that will affect the practicing rheumatologist.
Read ArticleDr. Woodcock Gives Senate FDA Update on Biosimilars
Biologics have been a great advance in therapeutics, but at great cost. Although biologics account for less than 1% of all prescriptions in the USA, they account for 28% percent of the cost of prescribed medications.
Read ArticleICD-10 Tips for Procrastinators
On October 1st, your coding memory will be getting a big and unwelcomed upgrade.
Even if the goverment shuts down, ICD-10 implementation goes forward.
Below are several sources to review what's ahead and what you should be focused on at this point.
Read ArticleThe Deadly Truth behind Paxil’s Study 329
Study 329 was published in 2001, and became a pivotal study for the anti-depressant drug Paxil (paroxetine). In 2002, over 2 million prescriptions were written for Paxil.
Read ArticleBiosimilar SB4 Performs Equally to Etanercept
Biosimilars are rapidly growing. Many of these will have their first introduction to countries outside of the USA. There is concern whether biosimilars will provide identical pharmacodynamics, biological function, efficacy and toxicity to reference products, both in the short and long term.
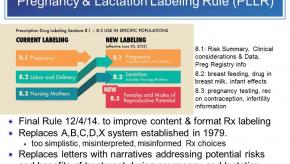
Read ArticlePLLR - The Pregnancy & Lactation Labeling Rule Explained
A recent article from Healio.com reviewed the FDA’s Pregnancy and Lactation Labeling Rule (PLLR) that became effective June 30th.
Read ArticleFuture 2 Trial Shows Secukinumab Efficacy in Psoriatic Arthritis
Secukinumab (Cosentyx) is an anti-IL-17 monoclonal antibody currently approved for use in moderate to severe psoriasis. It has also been studied in psoriatic arthritis (PsA) and shown to be safe and effective.
Read ArticleACR Position on MOC Highlighted in Newsweek
Newsweek has reported that the American Board of Internal Medicine has struggled in the last year with increasing criticism over its requirements for maintenance of certification, or MOC.
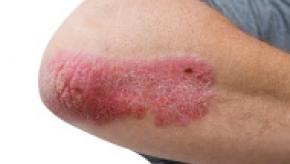
Read ArticleGout: New Classification Criteria from ACR/EULAR
The American College of Rheumatology and the European League against Rheumatism joined forces to finalize classification criteria for gout, a condition that affects 8.3 million Americans.
Read ArticleFunding for Rheumatology Research in Decline
The Rheumatology Research Foundation and Rheumatology News report that research projects funded by the National Institutes of Health (NIH) dropped by 52% from 2010 to 2014, while the number funded by private foundations fell by 29% over that period.
Read ArticleDrug Safety Reports & FDA Updates – August 2015
Safety reports from literature include long-term safety of rituximab, rituximab-induced neutropenia, tabalumab (Anti-BAFF Mab) results from the ILLUMINATE trial, colchicine toxicity increased in CKD patients, FDA updates and drug labeling changes, and more.
Read Article