All News
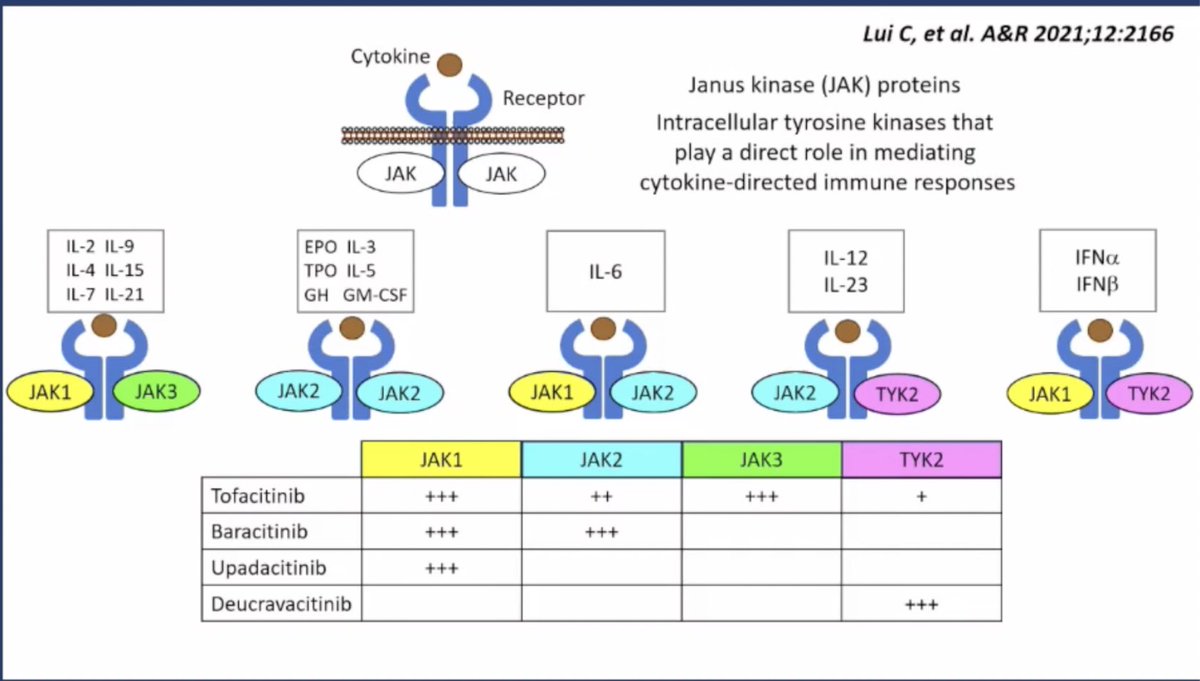
RA-ILD: an update
Interstitial lung disease (ILD) remains a leading cause of mortality in rheumatoid arthritis (RA). Estimates of involvement of ILD in RA remain relatively imprecise (estimated prevalence 5-20%), though this partly reflects the wide spectrum of ILD and differing ascertainment.
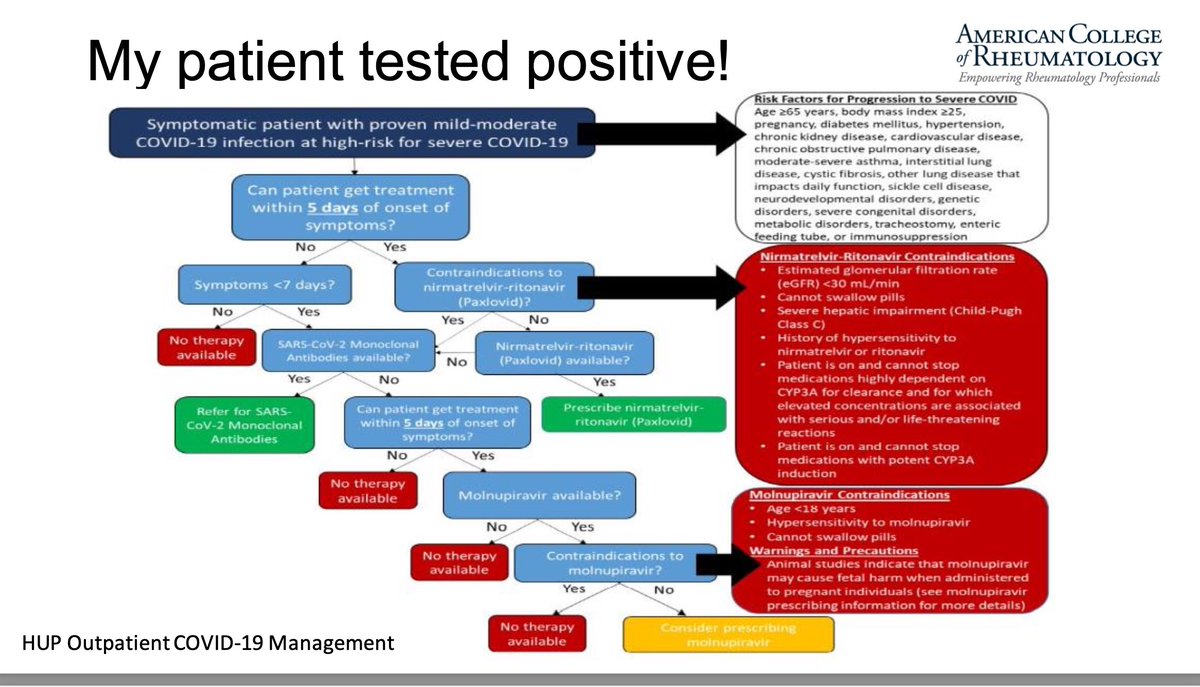
Read ArticleWhere are we with treating Pre-Rheumatoid Arthritis?
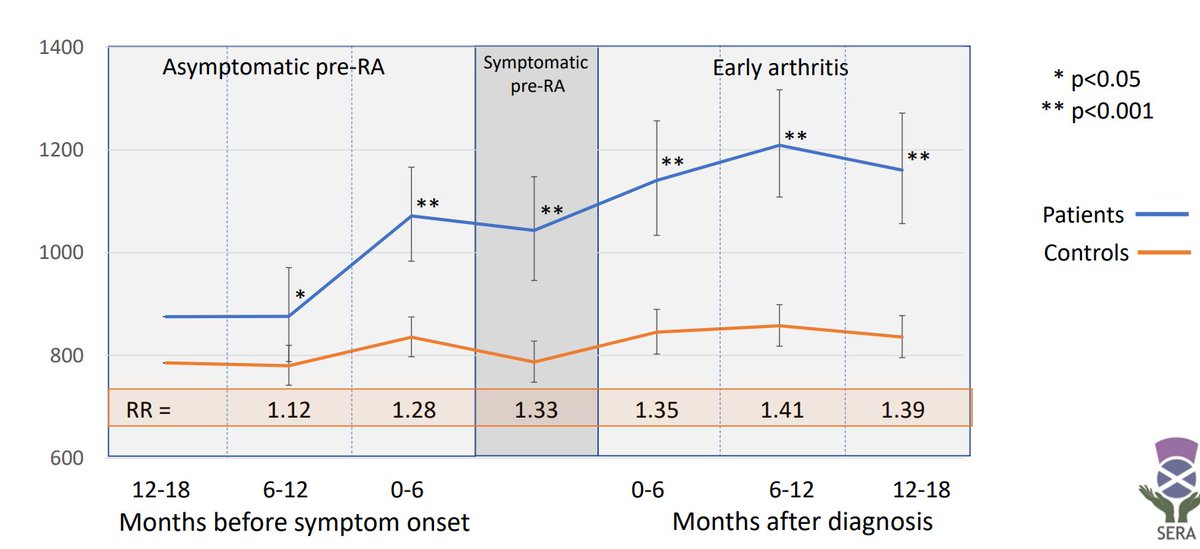
The ability to prevent RA in individuals at risk is a holy grail in rheumatology. There is a long history dating back to the PROMPT trial of methotrexate and PRAIRIE trial of rituximab. Both otrials showed an effect, but it seemed more likely to be a delaying of RA than prevention or modulation. Framing it another way, there were better outcomes in pre-RA because we were actually treating RA as it emerged with a proven effective treatment. It is in this setting that three trials of ongoing studies in RA preventative therapy are presented at ACR.
Read Article
Catherine Sims, MD DrCassySims ( View Tweet)
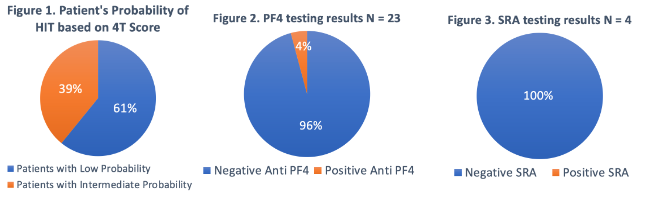
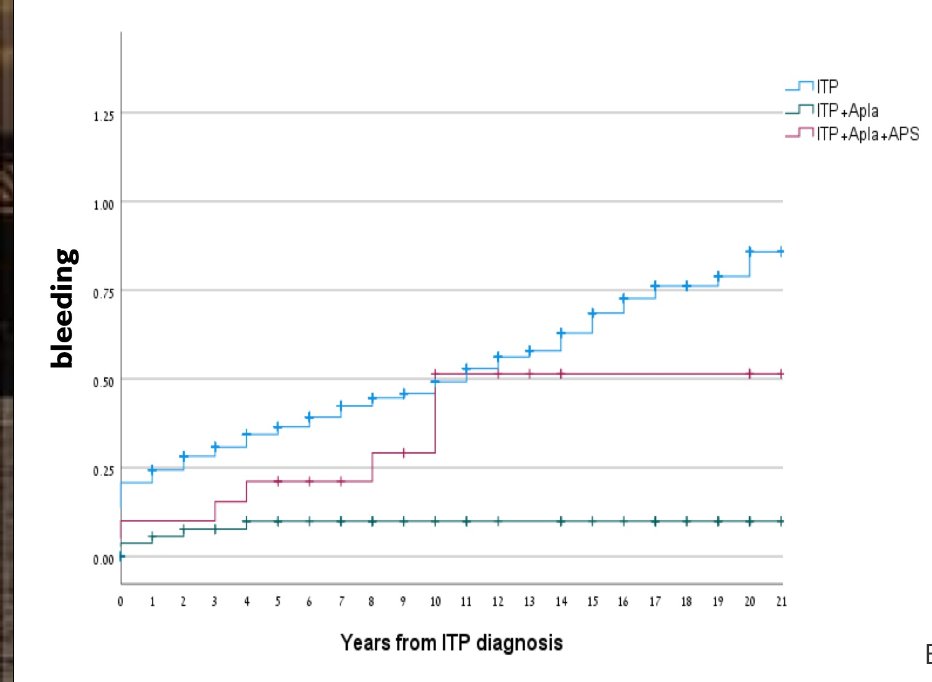

Dr. Rachel Tate uptoTate ( View Tweet)

Dr. Antoni Chan synovialjoints ( View Tweet)
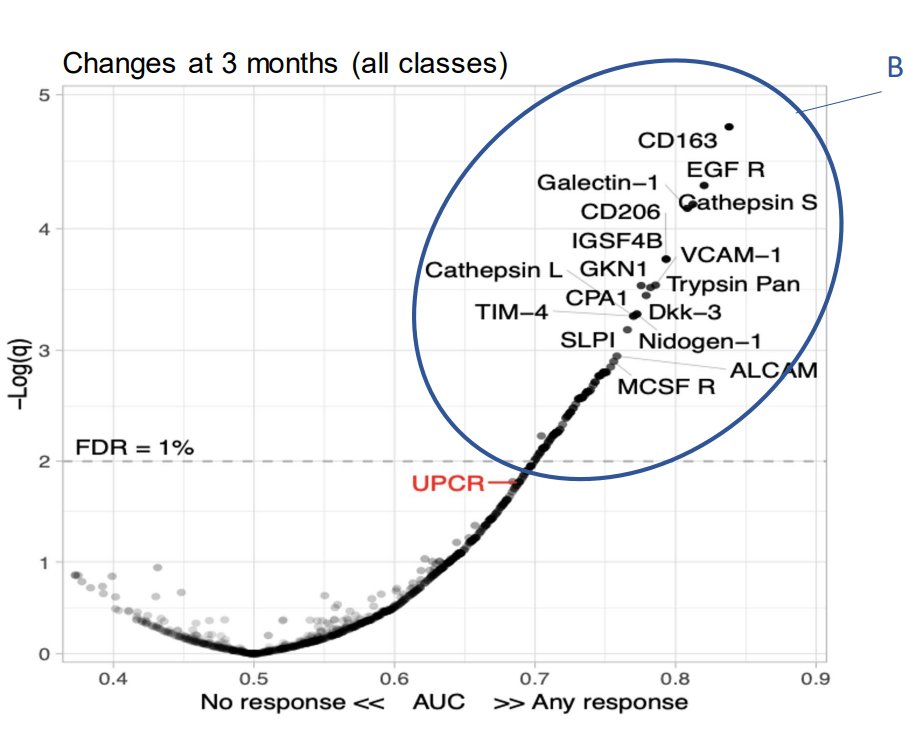

Dr. Rachel Tate uptoTate ( View Tweet)

Eric Dein ericdeinmd ( View Tweet)