Neurologic Events with TNF Inhibitor Therapy Save
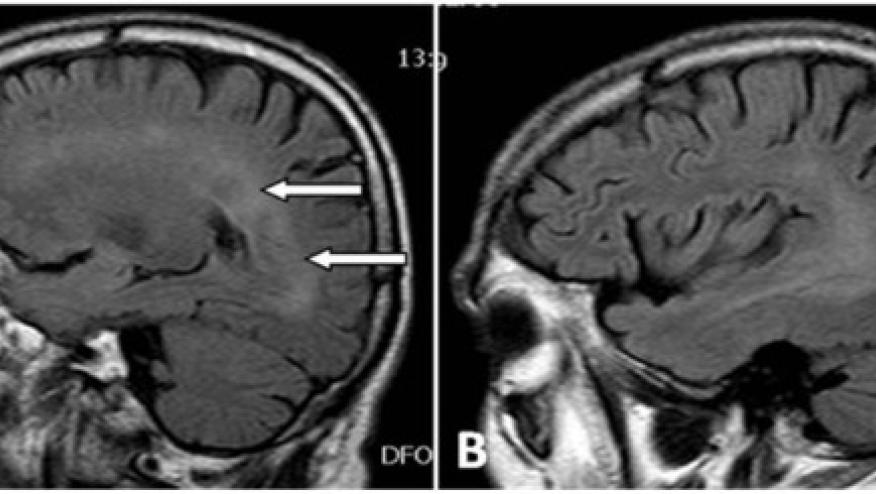
Demyelinating disorders are an uncommon complication of TNF inhibition and have been described with all TNF inhibitors but also with other biologics.
In this prospective evaluation of 77 patients with rheumatoid arthritis, psoriatic arthritis, ankylosing spondylitis and spondyloarthritis, neurologic evaluations were performed before and after anti-TNF therapy. Evaluations included brain and cervical MRI, neuropsychiatric testing and electrophysiologic studies were performed.
Two patients did not receive anti-TNFα therapy because brain MRIs at baseline revealed lesions compatible with demyelinating diseases. Thus, 75 patients received anti-TNFα (38 infliximab, 19 adalimumab and 18 etanercept). Three patients developed neurological adverse events - one each with optic neuritis, left facial nerve and MRI demyelinating lesions, and lower extremity tingling with a peripheral neuropathy.
Based on these studies, the estimate rate of neurological adverse events with anti-TNF therapy is 4% (3/75).










If you are a health practitioner, you may Login/Register to comment.
Due to the nature of these comment forums, only health practitioners are allowed to comment at this time.