Psoriatic Arthritis: Depressing News on Second- and Later-Line Treatments Save
Most patients with psoriatic arthritis (PsA) failed to achieve low disease activity after switching from one targeted therapy to another, real-world data from Scandinavian registries indicated.
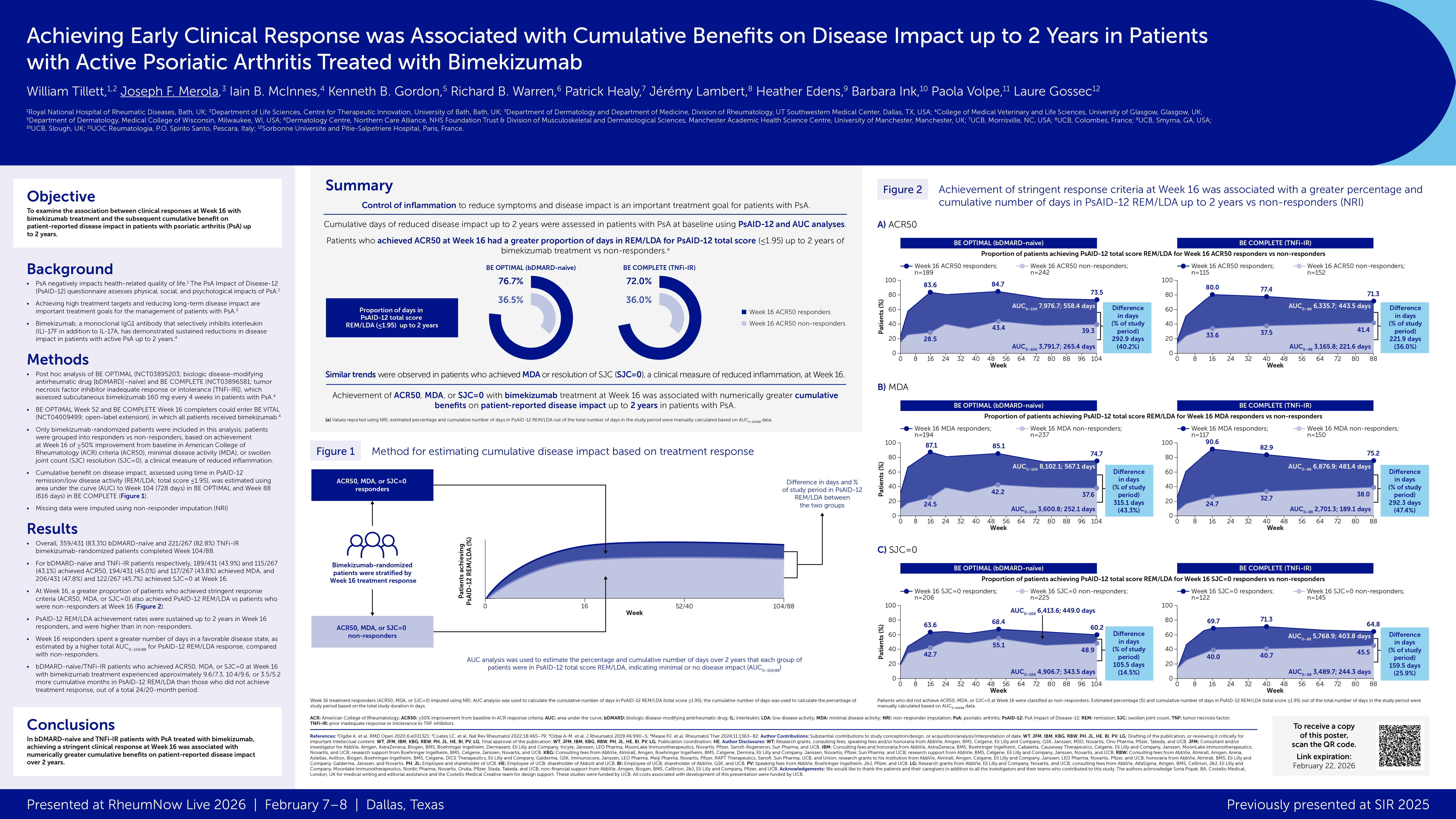
Moreover, patients became increasingly less likely to see substantial symptom improvement as they moved on to third-, fourth, and later-line treatments, reported Bente Glintborg, MD, PhD, of University Hospital of Copenhagen, and colleagues in the Annals of the Rheumatic Diseases.
Of all the treatments analyzed in the study -- seven in all, in more than 10,000 treatment courses given in Iceland, Norway, Sweden, Finland, and Denmark from 2012 to 2020 -- one stood out as more effective than the others. That was the tumor necrosis factor (TNF) inhibitor adalimumab (Humira). When it was given as second- or third-line treatment, Glintborg and colleagues found, 59% of PsA patients achieved low disease activity and 65% stayed on the drug for at least 1 year.
In contrast, fewer than half of patients using any of the other six agents, which were not TNF inhibitors, remained on them for a full year and even fewer achieved low disease activity.
Those agents were:
- Apremilast (Otezla)
- Ixekizumab (Taltz)
- Abatacept (Orencia)
- Ustekinumab (Stelara)
- Secukinumab (Cosentyx)
- Tofacitinib (Xeljanz)
And at fourth- or later-line, none of the seven drugs led to low disease activity (defined as a score of 14 or less on the 28-joint Disease Activity Index for Psoriatic Arthritis) in even 30% of patients, and 1-year retention rates were equally poor. Nevertheless, patients using non-TNF inhibitors appeared less likely to achieve low disease activity than those trying adalimumab for this type of salvage therapy (some comparisons showed nonsignificant trends in that direction while others were statistically significant).
"Superior outcomes for adalimumab indicate that the positioning of newer [biologic/targeted agents] in the PsA treatment algorithm remains to be established," the researchers concluded. Furthermore, "the poor remission rates irrespective of mode of action in biologic-experienced patients challenge the feasibility of treat-to-target approaches and pinpoint the need for additional treatment alternatives, including non-pharmacologic interventions."
The findings recall those from the landmark STAR*D trial of antidepressants opens in a new tab or window, which found that remission from depression became progressively less attainable when the first two treatment attempts failed. That study prompted a sea change opens in a new tab or window in how psychiatrists approached depression therapy.
Glintborg and colleagues undertook this study to fill in a major knowledge gap in PsA therapy: how the increasing multitude of available drugs stack up against one another. A few head-to-head trials have been conducted, but not enough to inform a proper treatment algorithm, and with inconsistent results to boot. Meta-analyses have also failed to establish clear efficacy differences, the researchers said.
"From an evidence-based perspective," the group noted, "it is not surprising that the 2019 EULAR (European Alliance of Associations for Rheumatology) recommendations adopted a cautious approach and refrained from ranking" the inhibitors of TNF, interleukin-17, and interleukin-12/23. The Janus-associated kinase inhibitor tofacitinib and apremilast and abatacept, whose mechanisms of action are less well understood, were recommended only in special cases, on the basis of limited data. Consequently, Glintborg and colleagues wanted to look at comprehensive registry data that could show how the drugs have been used in the real world.
They consulted PsA registers in each of the five Nordic countries, identifying 5,659 courses of treatment with adalimumab and 4,767 with the other six drugs. (Two other products, guselkumab [Tremfya] and upadacitinib [Rinvoq], are now approved for PsA but came on Scandinavian markets too late to provide meaningful data.)
Adalimumab was by far the most popular choice for first-line therapy (3,181 courses versus 500 or fewer for all the others) and for second- and third-line treatment as well (about half of the overall total). For fourth- or later-line treatment, however, secukinumab was used most commonly (719 courses out of 2,149, with adalimumab second at 398 and tofacitinib and ustekinumab following with just under 280 each).
All told, some 18% of patients tried four or more of the targeted agents studied, a sobering indication that a substantial number of PsA cases are refractory to current therapies. For these late-line treatments, Glintborg and colleagues noted that the number needed to treat (NNT) values were sometimes astonishingly high -- 63 for one of the agents, meaning that 62 patients had suboptimal responses for each one achieving low disease activity.
On the other hand, second- and third-line treatments appeared encouragingly effective, with an NNT=2 for adalimumab, and an NNT=3 for secukinumab, ixekizumab, tofacitinib, and ustekinumab.
Limitations to the study included differences in drug availability and treatment patterns among the five countries, the reliance on administrative data, and the multiplicity in PsA clinical presentations that complicates disease activity assessments.
Source Reference: opens in a new tab or windowGlintborg B, et al "Uptake and effectiveness of newer biologic and targeted synthetic disease-modifying antirheumatic drugs in psoriatic arthritis: results from five Nordic biologics registries" Ann Rheum Dis 2023; DOI: 10.1136/ard-2022-223650.









If you are a health practitioner, you may Login/Register to comment.
Due to the nature of these comment forums, only health practitioners are allowed to comment at this time.