News
Downside of Holding Methotrexate with COVID-19 Vaccination
A recent prospective, randomized, blinded clinical trial has shown that a 2 week hold of methotrexate (MTX) following COVID-19 (Sinovac-CoronaVac) vaccination resulted in greater anti-SARS-CoV-2 immunogenicity; but there was an increase in flare rates after the second dose of vaccine.
MMWR - NFL Study on When to Be Released if COVID(+)
MMWR has reported the results of the National Football Leagues (NFL) change in SARS-CoV-2 testing program that went into effect in December 2021, at the height of the Omicron variant surge, showing that among those with asymptomatic infection, only half were PCR test negative and could return to
Diagnostic Delay in Axial Spondyloarthritis (2.25.2022)
Dr. Jack Cush reviews the news and journal reports from the past week on RheumNow.com. This week, rheumatologic problems incited by respiratory infections, air pollution and how you live, now where you live.
COSMOS Study - Guselkumab Efficacy at 1 Year in Psoriatic Arthritis
Guselkumab, an IL-2319-subunit antibody, was studied in active psoriatic arthritis (PsA) patients who had a previously inadequate response (IR) to tumour necrosis factor inhibitors (TNFi) and after 1 year was shown to significantly improved joint and skin manifestations and physical function.
ACR Addresses Racial Disparities in Lupus Trials
The American College of Rheumatology (ACR) is launching two new initiatives to reduce racial disparities in lupus clinical trials: Training to Increase Minority Enrollment in Lupus Clinical Trials with CommunitY Engagement (TIMELY) and new Continuing Medical Educatio
Low COVID-19 Vaccine Risks in Rheumatic Patients
Data from the European Alliance of Associations for Rheumatology Coronavirus Vaccine registry shows that patients with with inflammatory or noninflammatory rheumatic and musculoskeletal disease have similar adverse event rates as the general public.
Medscape: Physician Burnout Report 2022
The annual Medscape Physician Burnout & Depression survey has been published for 2022 and Rheumatology burnout stats have improved, now ranking 10th (middle of the pack).
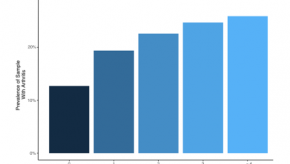
Increased Odds of Arthritis with Social Risk Factors
MMWR has published the results of the 2017 Behavioral Risk Factor Surveillance System showing that increases in the number of social risk factors independently increases the odds of arthritis and its burden in the USA.
RA: Still a Clinical Diagnosis
Positive Results from the DOA (Duloxetine in OsteoArthritis) Study
A pragmatic, open-label trial of osteoarthritis (OA) patients demonstrated that adding duloxetine treatment seems to be beneficial for end-stage knee OA patients with neuropathic-like symptoms (at risk of central sensitization).
RheumNow Podcast - RWCS 2022 Roundup (2.19.2022)
Drs. Jack Cush and Artie Kavanaugh review the highlights of the RWCS (Rheumatology Winter Clinical Symposium) from Maui.