Best of 2021: EULAR Guidelines on Intraarticular Therapy Save
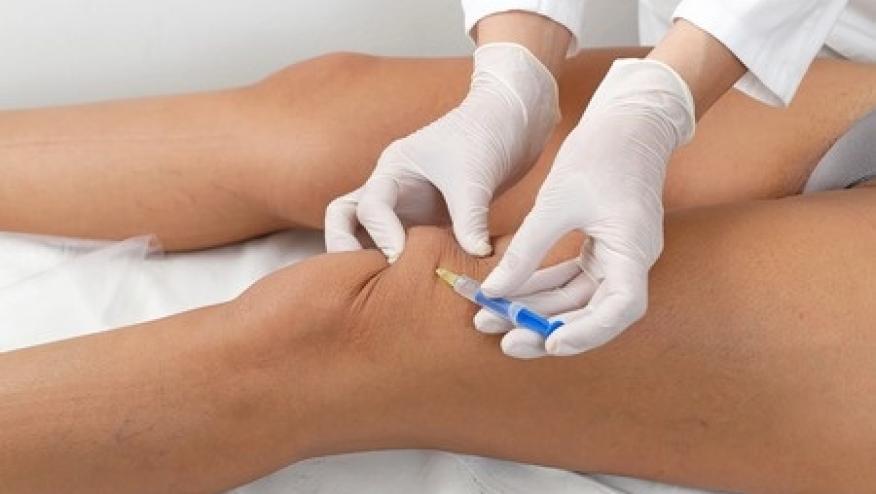
EULAR has published evidence-based recommendations on the use of intra-articular therapies (IAT) based on the literature review and recommendations of a multidisciplinary international task force. These IAT recommendations apply to adult patients with peripheral arthropathies.
The committee published 5 overarching principles and 11 recommendations addressing procedure and setting, accuracy, routine and special aseptic care, safety issues, precautions, special populations, repeated joint injections, local anaesthetics use and IAT aftercare.
Overarching principles
- IAT are recommended and widely used in the management of joint diseases
- The aim of IAT is to improve patient-centered outcomes
- Contextual factors are important and contribute to the effect of IAT
- IAT should be offered in the frame of full individualised information and a shared decision-making process
- A variety of health professionals perform these procedures routinely
Recommendations
- Patient must be fully informed of the nature of the procedure, the injectable, and potential benefits and risks; informed consent should be obtained and documented according to local habits
- The optimal setting for IAT includes: a) professional, clean, quiet, private, well-lightened room; b) Patient in an appropriate position, ideally on a couch/examination table, easy to lie flat; c) Equipment for aseptic procedures; d) Aid from another HP; and e) Resuscitation equipment close-by.
- Accuracy depends on the joint, route of entry, and health professional expertise; if available, imaging guidance, for example, ultrasound, may be used to improve accuracy.
- During pregnancy when injecting a joint one has to take into account whether the compound is safe for mother and baby
- Aseptic technique should always be undertaken when performing IAT
- Patients should be offered local anaesthetic explaining pros and cons
- Diabetic patients, especially those with suboptimal control, should be informed about the risk of transient increased glycaemia following IA GC and advised about the need to monitor glucose levels particularly from first to third day
- IAT is not a contraindication in people with clotting/bleeding disorders or taking antithrombotic medications, unless bleeding risk is high
- IAT may be performed at least 3 months prior to joint replacement surgery, and may be performed after joint replacement following consultation with the surgical team
- The shared decision to reinject a joint should take into consideration benefits from previous injections and other individualised factors (eg, treatment options, compound used, systemic treatment, comorbidities…)
- Avoid overuse of injected joints for 24 hours following IAT; however, immobilisation is discouraged.
Editor's note: This article was originally published May 26, 2021, and is being republished today as part of RheumNow's Best of 2021.










If you are a health practitioner, you may Login/Register to comment.
Due to the nature of these comment forums, only health practitioners are allowed to comment at this time.