EXXELERATE: New Thoughts on High RF Titer Save
When managing moderate-to-severely active rheumatoid arthritis (RA), it is also important to consider patients with elevated and high rheumatoid factor (RF) levels—they may have unique therapeutic needs and responses.1
We’ve convened two leading rheumatologists to provide their insights on the significance of RF levels in determining treatment options for patients with RA. They also discuss their perspectives on how CIMZIA® (certolizumab pegol) may help patients living with moderate-to-severely active RA with higher RF levels.
MEET THE RHEUMATOLOGISTS

Erin L. Arnold, MD, FACR
Rheumatologist
Skokie, IL

Soha Dolatabadi, MD
Rheumatologist
Los Angeles, CA
Focusing on Rheumatoid Factor
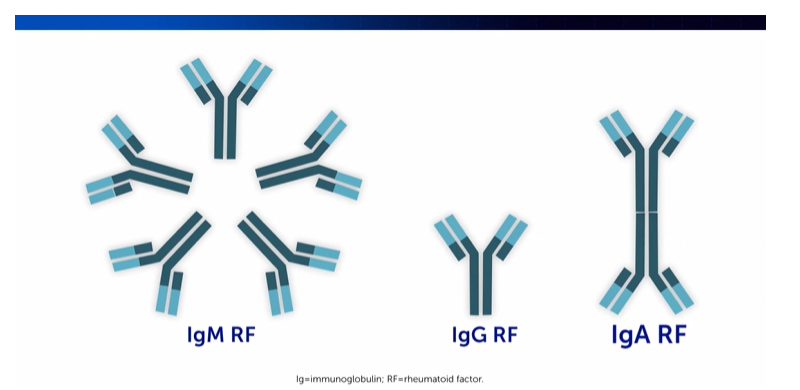
RF is comprised of immune system molecules that exist in the forms of IgM RF, IgG RF, and IgA RF.2,3 In the diagnosis and management of RA, RF is one of the many antibodies used in diagnostic workups and prognosis.4 Specifically, IgM is the most commonly tested form of RF in clinical practice.2
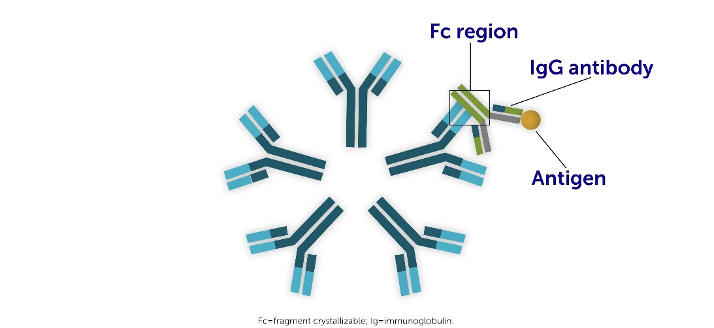
RF plays a central role in the pathophysiology of RA:
- RF, as an autoantibody, is associated with chronic inflammation in the body,2 and in the context of RA, the presence of RF has been linked to cytokine-dependent inflammation2,5
- RF neutralizes IgG molecules by binding to the fragment crystallizable (Fc) domains5
- RF can bind up to ten IgG1-Fc regions5
What High RF Levels Can Mean for Your Patients with RA
As a prognostic indicator, RF can provide us with a better understanding of RA disease severity, the risk of progression, and how a patient might respond to certain treatments.6,7,8
A majority of patients with RA are positive for RF and it can be present at varying levels – with most having higher RF levels at baseline, as you can see below:9
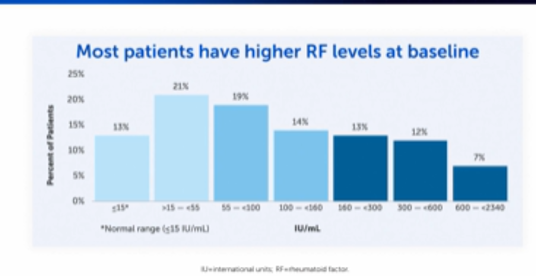
Moreover, we also know that RF levels can impact disease burden.2,5 Patients with higher RF levels are at risk of experiencing more aggressive and destructive forms of RA.2,5,8
If your patient has higher RF levels, they may be more likely to experience symptoms including:8,10
A Treatment Option for RA Across RF Levels
With 17 years of clinical use, CIMZIA is an FDA-approved treatment option that has delivered outcomes across different clinical manifestations in patients with moderate-to-severely active RA.11 In the RAPID 1 pivotal study, 59% of patients treated with CIMZIA 200 mg Q2W + MTX (n=393) vs. 14% of patients taking placebo + MTX (n=199) achieved ACR20 at Week 24.11,12
Learn more about considering patient characteristics and how CIMZIA may be used for patients with different RF levels:
As you weigh the risks and benefits of CIMZIA for your patients, please see the Important Safety Information below and link to full Prescribing Information with Boxed Warning regarding serious infections, including tuberculosis, and malignancies.11
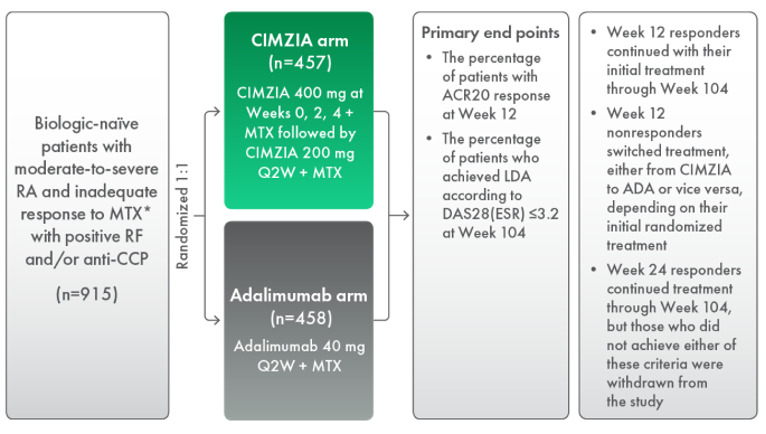
Results from EXXELERATE: A Phase 4 Head-to-Head Superiority Study
A Phase 4 head-to-head superiority study, EXXELERATE, evaluated the short- and long-term efficacy of CIMZIA compared with adalimumab, both in combination with methotrexate, in the treatment of patients with moderate-to-severely active RA who had not responded adequately to methotrexate.13
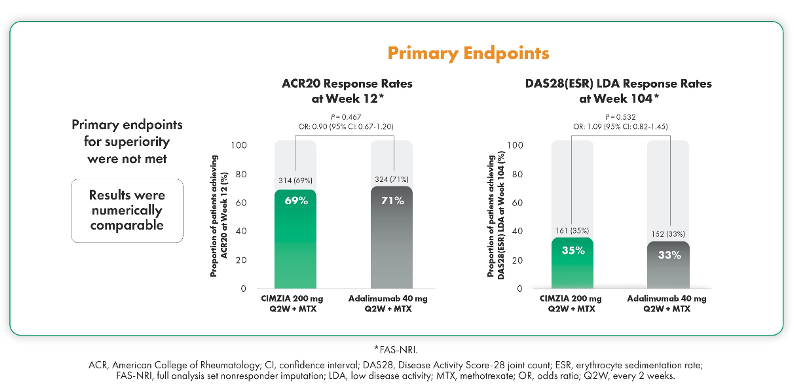
In EXXELERATE, the primary endpoints of superiority were not met. However, the results showed that CIMZIA and adalimumab were numerically comparable.13
EXXELERATE Post Hoc Analysis
Findings from a post hoc analysis conducted by Dr. Josef Smolen, Division of Rheumatology, Department of Medicine, Medical University of Vienna, Vienna, Austria, suggested that CIMZIA-treated patients achieved remission or LDA regardless of RF levels at Week 104.14 CIMZIA drug concentration was consistent in all patients for up to 2 years.14
Additionally, CIMZIA-treated patients achieved remission or low disease activity regardless of RF levels. Within the adalimumab subgroups, results were numerically lower in patients with higher RF levels at Week 104.14
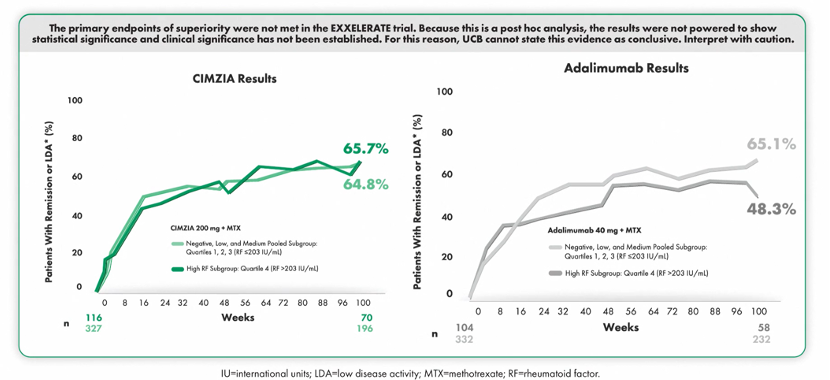
The primary endpoints of superiority were not met in the EXXELERATE trial. Because this is a post hoc analysis, the results were not powered to show statistical significance and clinical significance has not been established. For this reason, UCB cannot state this evidence as conclusive. Interpret with caution.14
EXXELERATE also examined drug pharmacokinetics in CIMZIA- and adalimumab-treated patients. Through week 104:14
- In patients treated with CIMZIA, drug plasma concentrations were similar across all RF subgroups14
- In the adalimumab subgroups, drug concentrations were lower in patients with higher RF versus lower levels of RF14
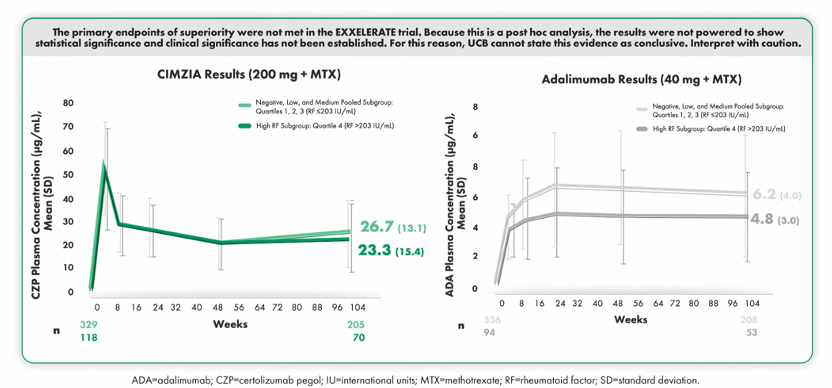
The primary endpoints of superiority were not met in the EXXELERATE trial. Because this is a post hoc analysis, the results were not powered to show statistical significance and clinical significance has not been established. For this reason, UCB cannot state this evidence as conclusive. Interpret with caution.14
These data from EXXELERATE suggested that CIMZIA delivered clinical outcomes regardless of baseline RF levels.14
Please see full Prescribing Information or visit cimziahcp.com to learn more about these results.
Research continues to highlight the implications of higher levels of RF in treating and managing patients with RA and the potential value of considering a tailored and personalized approach. In patients with RA, higher RF levels are considered a poor prognostic factor associated with more aggressive and destructive disease, higher disease activity, higher cardiovascular risk, and a higher risk of radiographic progression.9,10 As more is uncovered in this area, we are eager to discover new ways to improve patient outcomes across RF levels.
+++
CIMZIA is a tumor necrosis factor (TNF) blocker indicated for treatment of adults with moderate-to-severely active rheumatoid arthritis.
###
Important Safety Information you should know about CIMZIA® (certolizumab pegol)
CONTRAINDICATIONS
CIMZIA is contraindicated in patients with a history of hypersensitivity reaction to certolizumab pegol or to any of the excipients. Reactions have included angioedema, anaphylaxis, serum sickness, and urticaria.
SERIOUS INFECTIONS
Patients treated with CIMZIA are at increased risk for developing serious infections that may lead to hospitalization or death. Most patients who developed these infections were taking concomitant immunosuppressants such as methotrexate or corticosteroids.
Discontinue CIMZIA if a patient develops a serious infection or sepsis.
Reported infections include:
- Active tuberculosis (TB), including reactivation of latent TB. Patients with TB have frequently presented with disseminated or extrapulmonary disease. Test patients for latent TB before CIMZIA use and during therapy. Initiate treatment for latent TB prior to CIMZIA use.
- Invasive fungal infections, including histoplasmosis, coccidioidomycosis, candidiasis, aspergillosis, blastomycosis, and pneumocystosis. Patients with histoplasmosis or other invasive fungal infections may present with disseminated, rather than localized, disease. Antigen and antibody testing for histoplasmosis may be negative in some patients with active infection. Consider empiric anti-fungal therapy in patients at risk for invasive fungal infections who develop severe systemic illness.
- Bacterial, viral, and other infections due to opportunistic pathogens, including Legionella and Listeria.
Carefully consider the risks and benefits of treatment with CIMZIA prior to initiating therapy in the following patients: with chronic or recurrent infection; who have been exposed to TB; with a history of opportunistic infection; who resided in or traveled in regions where mycoses are endemic; with underlying conditions that may predispose them to infection. Monitor patients closely for the development of signs and symptoms of infection during and after treatment with CIMZIA, including the possible development of TB in patients who tested negative for latent TB infection prior to initiating therapy.
- Do not start CIMZIA during an active infection, including localized infections.
- Patients older than 65 years, patients with co-morbid conditions, and/or patients taking concomitant immunosuppressants may be at greater risk of infection.
- If an infection develops, monitor carefully and initiate appropriate therapy.
MALIGNANCY
Lymphoma and other malignancies, some fatal, have been reported in children and adolescent patients treated with TNF blockers, of which CIMZIA is a member. Consider the risks and benefits of CIMZIA treatment prior to initiating or continuing therapy in a patient with known malignancy.
- In clinical trials, more cases of malignancies were observed among CIMZIA-treated patients compared to control patients.
- In CIMZIA clinical trials, there was an approximately 2-fold higher rate of lymphoma than expected in the general U.S. population. Patients with rheumatoid arthritis, particularly those with highly active disease, are at a higher risk of lymphoma than the general population.
- Malignancies, some fatal, have been reported among children, adolescents, and young adults being treated with TNF blockers. Approximately half of the cases were lymphoma, while the rest were other types of malignancies, including rare types associated with immunosuppression and malignancies not usually seen in this patient population.
- Postmarketing cases of hepatosplenic T-cell lymphoma (HSTCL), a rare type of T-cell lymphoma, have been reported in patients treated with TNF blockers, including CIMZIA. These cases have had a very aggressive disease course and have been fatal. The majority of reported TNF blocker cases have occurred in patients with Crohn’s disease or ulcerative colitis, and the majority were in adolescent and young adult males. Almost all of these patients had received treatment with azathioprine or 6-mercaptopurine concomitantly with a TNF blocker at or prior to diagnosis. Carefully assess the risks and benefits of treating with CIMZIA in these patient types.
- Cases of acute and chronic leukemia were reported with TNF blocker use.
HEART FAILURE
- Worsening and new onset congestive heart failure (CHF) have been reported with TNF blockers. Exercise caution and monitor carefully.
HYPERSENSITIVITY
- Angioedema, anaphylaxis, dyspnea, hypotension, rash, serum sickness, and urticaria have been reported following CIMZIA administration. If a serious allergic reaction occurs, stop CIMZIA and institute appropriate therapy. The needle shield inside the removable cap of the CIMZIA prefilled syringe contains a derivative of natural rubber latex that may cause an allergic reaction in individuals sensitive to latex.
HEPATITIS B VIRUS REACTIVATION
- Use of TNF blockers, including CIMZIA, may increase the risk of reactivation of hepatitis B virus (HBV) in patients who are chronic carriers. Some cases have been fatal.
- Test patients for HBV infection before initiating treatment with CIMZIA.
- Exercise caution in patients who are carriers of HBV and monitor them before and during CIMZIA treatment.
- Discontinue CIMZIA and begin antiviral therapy in patients who develop HBV reactivation. Exercise caution when resuming CIMZIA after HBV treatment.
NEUROLOGIC REACTIONS
- TNF blockers, including CIMZIA, have been associated with rare cases of new onset or exacerbation of central nervous system and peripheral demyelinating diseases, including multiple sclerosis, seizure disorder, optic neuritis, peripheral neuropathy, and Guillain-Barré syndrome.
HEMATOLOGIC REACTIONS
- Rare reports of pancytopenia, including aplastic anemia, have been reported with TNF blockers. Medically significant cytopenia has been infrequently reported with CIMZIA.
- Consider stopping CIMZIA if significant hematologic abnormalities occur.
DRUG INTERACTIONS
- Do not use CIMZIA in combination with other biological DMARDS.
AUTOIMMUNITY
- Treatment with CIMZIA may result in the formation of autoantibodies and, rarely, in development of a lupus-like syndrome. Discontinue treatment if symptoms of a lupus-like syndrome develop.
IMMUNIZATIONS
- Avoid use of live vaccines during or immediately prior to initiating CIMZIA. Update immunizations in agreement with current immunization guidelines prior to initiating CIMZIA therapy.
ADVERSE REACTIONS
- The most common adverse reactions in CIMZIA clinical trials (≥8%) were upper respiratory infections (18%), rash (9%), and urinary tract infections (8%).
US-CZ-2400615
Date of preparation: February 2025
CIMZIA® is a registered trademark of the UCB Group of Companies.
All other trademarks are the property of their respective holders.
©2025 UCB, Inc., Smyrna, GA 30080. All rights reserved.
References:
- Tiwari V, Jandu JS, Bergman MJ. Rheumatoid Factor. [Updated 2023 Jul 24]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK532898/
- Ingegnoli F, Castelli R, Gualtierotti R. Rheumatoid factors: clinical applications. Dis Markers. 2013;35(6):727-734.
- Schroeder HW Jr, Cavacini L. Structure and function of immunoglobulins. J Allergy Clin Immunol. 2010;125(2 Suppl 2):S41-S52.
- Aletaha D, et al. 2010 Rheumatoid arthritis classification criteria: an American College of Rheumatology/European League Against Rheumatism collaborative initiative. Arthritis Rheum. 2010;62(9):2569-2581.
- Tanaka Y, Takeuchi T, Haaland D, et al. Efficacy of certolizumab pegol across baseline rheumatoid factor subgroups in patients with rheumatoid arthritis: post-hoc analysis of clinical trials. Int J Rheum Dis. 2023;26(7):1248-1259.
- Thomas J, et al. Personalized medicine in rheumatoid arthritis: Combining biomarkers and patient preferences to guide therapeutic decisions. Best Prac Res Clin Rheumatol. 2022;36(4):101812.
- Julia A, et al. Interactions between rheumatoid arthritis antibodies are associated with the response to anti-tumor necrosis factor therapy. BMC Musculoskel Dis. 2021;22(372):1-7.
- Nell VPK, Machold KP, Stamm TA, et al. Autoantibody profiling as early diagnostic and prognostic tool for rheumatoid arthritis. Ann Rheum Dis. 2005;64(12):1731-1736.
- Takeuchi T, et al. High titers of both rheumatoid factor and anti-CCP antibodies at baseline in patients with rheumatoid arthritis are associated with increased circulating baseline TNF level, low drug levels, and reduced clinical responses: a post hoc analysis of the RISING study. Arthritis Res Ther. 2017;19(1):194.
- Fazeli MS, Khaychuk V, Wittstock K, et al. Cardiovascular disease in rheumatoid arthritis: risk factors, autoantibodies, and the effect of antirheumatic therapies. Clin Med Insights Arthritis Musculoskelet Disord. 2021;14:11795441211028751. doi:10.1177/1179541211028751.
- CIMZIA® Prescribing Information. Available at www.ucb-usa.com/cimzia-prescribing-information.pdf.
- Keystone E, van der Heijde D, Mason D Jr, et al. Certolizumab pegol plus methotrexate is significantly more effective than placebo plus methotrexate in active rheumatoid arthritis: findings of a fifty-two-week, phase III, multicenter, randomized, double-blind, placebo-controlled, parallel-group study. Arthritis Rheum. 2008;58(11):3319-3329.
- Smolen JS, Burmester GR, Combe B, et al. Head-to-head comparison of certolizumab pegol versus adalimumbab in rheumatoid arthritis: 2-year efficacy and safety results from the randomised EXXELERATE study. Lancet. 2016;388(10061):2763-2774.
- Smolen JS, Taylor PC, Tanaka Y, et al. Impact of high rheumatoid factor levels on treatment outcomes with certolizumab pegol and adalimumab in patients with rheumatoid arthritis. Rheumatology (Oxford). 2024;63(11):3015-3024. doi:10.1093/rheumatology/keae435.