Variability of Guidelines on the Perioperative Use of DMARDs in Rheumatic Diseases Patients Save
More effective treatments in patients with rheumatic diseases have resulted in less need for major surgery, yet a substantial number of RMD patients will undergo surgery often in the setting of disease-modifying anti-rheumatic drugs (DMARDs) use. A scoping review looked at numerous clinical practice guidelines/recommendations for the perioperative management of DMARDs and found inconsistencies and low quality evidence, with great reliance on conditional (expert opinion) recommendations.
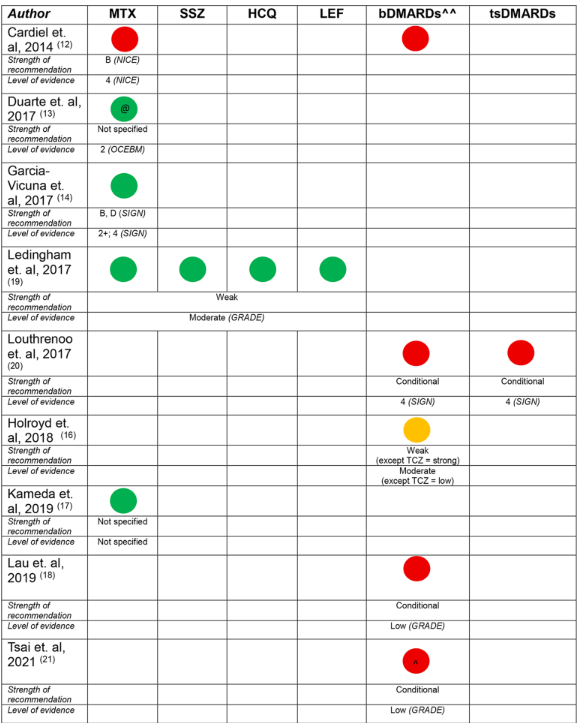
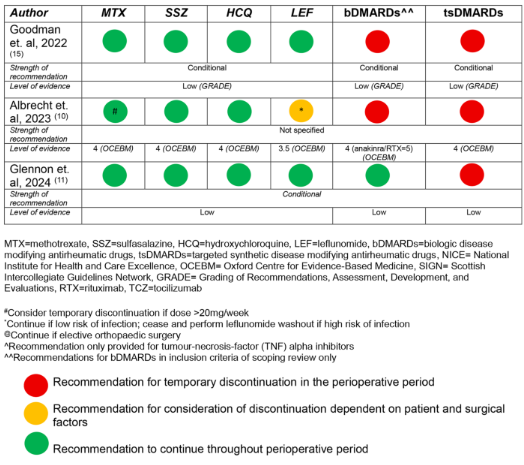
A literature identified guidelines published since 2014 by national/international academic societies in rheumatology addressing perioperative management of DMARDs in any RMD (RA, AS, PsA, JIA, SLE, etc). From 120 articles, the review was narrowed to 12 articles, with 12 corresponding multinational guidelines.
Although guidelines for the use of DMARDs in the perioperative period are widely available, the development process and recommendations vary between guidelines. There is a lack of high quality evidence to support recommendations for non-elective, non-orthopaedic surgery cases. Variations in recommendations for bDMARDs in the perioperative period were common, potentially leading to more practice variation in bDMARD use in the perioperative period.
Low-moderate quality evidence supported these recommendations, based on evidence from studies of participants undergoing elective orthopaedic surgery. Guidelines varied in development process, format, the choice of evidence system, level of evidence, strength of recommendation and recommendations for biologic DMARD (bDMARD) use and timing of surgery.
Recommendations regarding conventional synthetic DMARDs (csDMARDs)
Only 8 guidelines had recommendations for the perioperative management of csDMARDs, and not all csDMARDs were considered - hydroxychloroquine in 4; sulfasalazine in 4; leflunomide in 4; and methotrexate in 8 (100%).
All guidelines recommended the continuation of hydroxychloroquine and sulfasalazine (‘do not routinely discontinue’). DMARD continuation was recommened by 75% for leflunomide and 88% for methotrexate (1 guideline suggested holding methotrexate for 1 week prior to operative intervention).
The strength of the recommendation was weak/conditional in 50% and unspecified in 40%. There was low certainty of evidence underlying each of the recommendations, and a particular absence of evidence for recommendations in non-elective, non-orthopaedic surgery cases. perative
Recommendations regarding biological DMARDs (bDMARDs)
Recommendations regarding the perioperative bDMARDs use was included in 8 guidelines. Temporary bDMARD discontinuation was conditionally recommended in 75%. Recommendations for duration of temporary discontinuation of bDMARDs were highly variable, including: ‘3–5 half lives’, ‘discontinue for 2 weeks before or after surgery’, ‘1–2 months before surgery anti-TNF should be discontinued’, ‘time surgery at the end of dosing cycle’, or ‘discontinue one dosing cycle prior’. For rituximab, the recommendation for surgical timing ranged from 3 to 7 months.
Recommendations for the recommencement of bDMARDs following surgery was generally based on wound healing and absence of infection, with 38% conditionally recommending recommencement 14 days post-operatively, though the evidence for this recommendation was noted to be of very low quality.
Overall, the strength of the recommendation was weak/conditional in 63%, and unspecified in 25%. All of the recommendations were based on low certainty evidence from case series or other observational studies.
Recommendations regarding targeted synthetic DMARDs (tsDMARDs)
Five guidelines had recommendations regarding tsDMARDs, with a conditional recommendation in 80% (4) guidelines. Patient and surgical factors were considered to be important factors with temporary discontinuation in the perioperative period. The timing of temporary discontinuation ranged from 3 to 7 days prior to planned surgery, and recommencing 4–14 days post operatively. Wound healing and absence of infection were described as potential determinants when considering recommencement.
Recommendations for the use of glucocorticoids
Recommendations on glucocorticoids were only included in 3 (25%) guidelines - none recommending temporary discontinuation. Two of the three guidelines recommended against stress dosing.










If you are a health practitioner, you may Login/Register to comment.
Due to the nature of these comment forums, only health practitioners are allowed to comment at this time.