All News
The RheumNow Week in Review – 28 July 2017
Dr. Jack Cush reviews the news and articles from the past week on RheumNow.com:
Read ArticleBiosimilar Pricing Wars Have Begun
Despite the approval of four anti-TNF biosimilars in 12 months, their introduction into the U.S. market has been slow and - until now - with paultry discounts compared to their introduction throughout Europe, where discounts averaged 50% and were as high as 70% (compared to the price of Remicade).
Read ArticleBaricitinib Derailed by FDA Review
Eli Lilly and Co disclosed today that upon further discussions with the US Food and Drug Administration (FDA) there would be a delay in further regulatory decisions regarding baricitinib, a JAK inhibitor, that is being developed for use in rheumatoid arthritis (RA).
Read ArticleBenlysta FDA Approved for Sub-Q Use in Lupus
The US Food and Drug Administration (FDA) has approved a new subcutaneous formulation of Benlysta (belimumab) for use in active, autoantibody‑positive Systemic Lupus Erythematosus (SLE) patients.
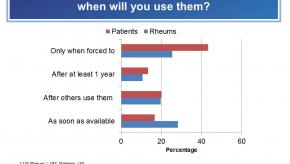
Read ArticleRheumatologists and Patients Concerns over Biosimilars - RheumNow “Live Vote” Results
The RheumNow “Live Vote” on the use, uptake and safety of biosimilars contrasts rheumatologist and patient views. Despite FDA approval and looming introduction, there is considerable concern, knowledge gaps and hesitancy regarding their uptake in the US. The survey's primary question demonstrates that less than one-third of US rheumatologists are prepared to adopt biosimilars when they are available.
Read ArticleThe RheumNow Week in Review – 21 July 2017
Dr. Jack Cush reviews the news from the past week on RheumNow.com:
Read ArticleCumulative Genetic Hit Hypothesis for Lupus
A recent report in Nature Communications has studied multiple nationalities has shown a "disentangled complex HLA multigenic effect" underlying the genetic basis of systemic lupus erythematosus (SLE).
Read ArticleFDA Approves Tremfya (guselkumab) for Plaque Psoriasis
The U.S. Food and Drug Administration (FDA) has approved the IL-23 inhibitor, Tremfya (guselkumab), for patients with moderate to severe plaque psoriasis.
Read ArticleThe RheumNow Week in Review – 14 July 2017
Dr. Jack Cush reviews highlights from the past week on RheumNow.com:
Read ArticlePharma Pays $8.2 Billion to Physicians in 2016
Data made public by the Centers for Medicare & Medicaid Services shows that drug and device makers made nearly $8.2 billion in payments to U.S. physicians and teaching hospitals in the last year. Since 2013, CMS has been tracking physician payments as part of the Affordable Care Act.
Read ArticleOrencia Approved for Use in Psoriatic Arthritis
The FDA has approved both IV and SC ORENCIA (abatacept) for the treatment of adults with active Psoriatic Arthritis.
Read ArticleACR Says Senate Healthcare Bill Falls Short of Protecting Americans with Rheumatic Disease
Dr. Sharad Lakhanpal, President of the American College of Rheumatology has responded to the Senate's proposed changes to healthcare coverage in the USA.
Read ArticleHighlights from EULAR 2017 - From RheumReports.com
Dr. Janet Pope reviews her favorite and least favorite presentations and abstracts from EULAR 2017.
Read ArticleDrug Costs Vary by 600% in Different Countries
The Canadian Medical Association Journal has studied the costs of prescription drugs in 10 high-income countries (seven European countries, Australia, Canada and New Zealand) and found that many of these medicines varied by more than 600%.
Read ArticleTildrakizumab, an IL-23 Inhibitor, is Successful in Plaque Psoriasis
Therapeutic options for cutaneous psoriasis (and psoriatic arthritis as well) are rapidly expanding. Lancet has published the impressive results of two phase III trials of tildrakizumab, a IgG1 antibody against interleukin 23 p19, in patients with active chronic plaque psoriasis.
Read ArticleEULAR 2017 – Day 3 Highlights
Friday was a mega-day in Madrid as this day all the late-breaking abstracts were presented, in addition to the bulk of high-impact podium presentations in several areas.
Here are the major presentations from several clinical areas.
Read ArticleLancet Launches EULAR 2017 and "A Platinum Age of Rheumatology"
The current Lancet edition for 10 June 2017 is a rheumatology rich collection.
Read ArticleMixed Results with Combination Therapy in Gout
Lesinurad, a selective URAT-1 inhibitor has been approved for coadministration with a urate-lowering therapy (ULT) in patients with gout for nearly a year. The CRYSTAL study was one of the studies that lead to FDA approval.
Read ArticleSupreme Court Decision Favors Earlier Biosimilar Adoption
On Monday June 11th, the US Supreme Court unanimously ruled that biosimilar manufacturers can bring their drugs to market faster by eliminating the provision that the biosimar manufacturer had to give the innovator company 180 days notice before launching the new biosimilar.
Read ArticleFDA Requests Removal of OPANA-ER from the Market
The U.S. Food and Drug Administration has asked Endo International to withdraw Opana ER from the market, stating that benefits no longer outweigh its risks of this long-acting opioid.
Read Article