All News
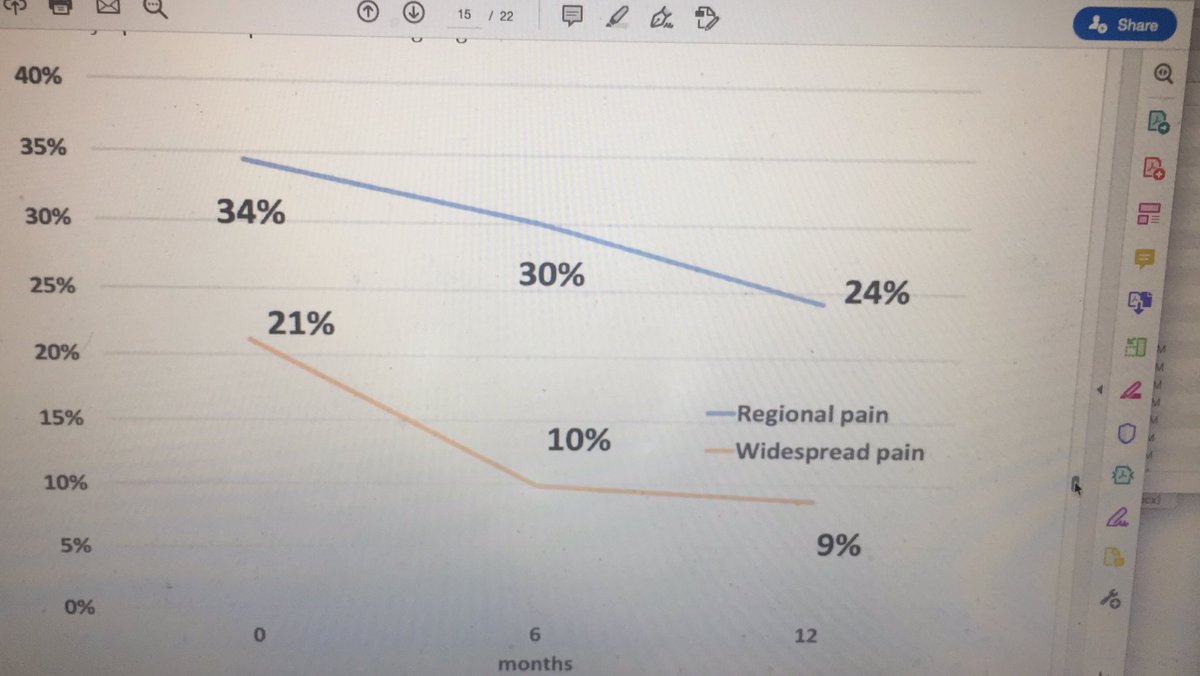
Early RA pts may develop chronic widespread pain. Not all have fibromyalgia. Multiple areas of pain & inflammation ^ chronic pain & other factors ie women high initial pain longer symptoms @CRASCRRheum @RheumNow #ACR20 abstr#187 1/3 at onset have regional pain 1/4 at 1yr https://t.co/7qOxH4h7Dd https://t.co/IFO1uwyR0o
Janet Pope Janetbirdope ( View Tweet)
#ACR20 Basic Science Year-in-review Really important work by @danaorange showing PRIME cells can predict flares in #RheumatoidArthritis patients #HSSrheum @RheumNow
https://t.co/XTqqinAN2a https://t.co/24HkqgHS0H
Bella Mehta bella_mehta ( View Tweet)

#ACR20 year in review by @JYazdanyMD latest data PEXIVAS and SEMIRA trials on corticosteroids - would you change your clinical practice to minimize even low dose Corticosteroids in RA and Vasculitis? @RheumNow
https://t.co/l7PTyANpjq
https://t.co/MRGbsjaGae
Bella Mehta bella_mehta ( View Tweet)

The #ACR20 Great Debate is on! One issue point is lab monitoring on TNF vs JAK. What's your take? @RheumNow
Eric Dein ejdein1 ( View Tweet)
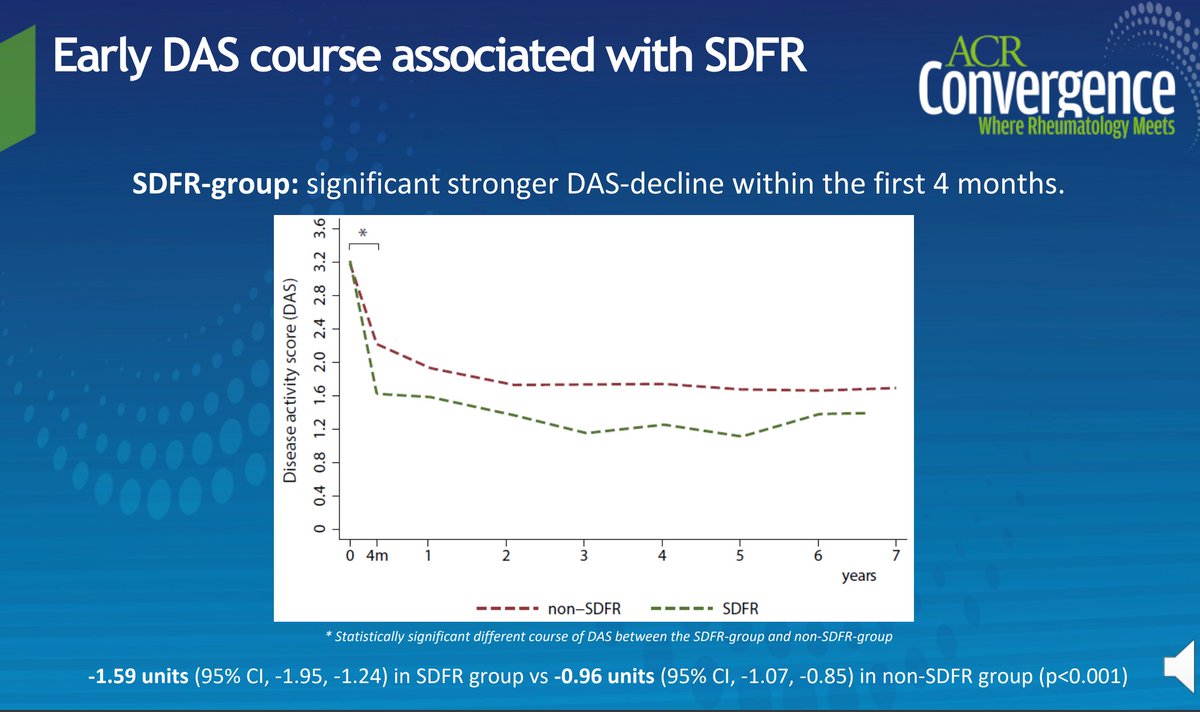
Who can have sustained DMARD-free remission (SDFR)? Abst#0479 says early DAS response after DMARD initiation in ACPA-negative patients #ACR20
@RheumNow. Who do you try for DMARD-free intervals? https://t.co/EOfPt4YQAu
Eric Dein ejdein1 ( View Tweet)
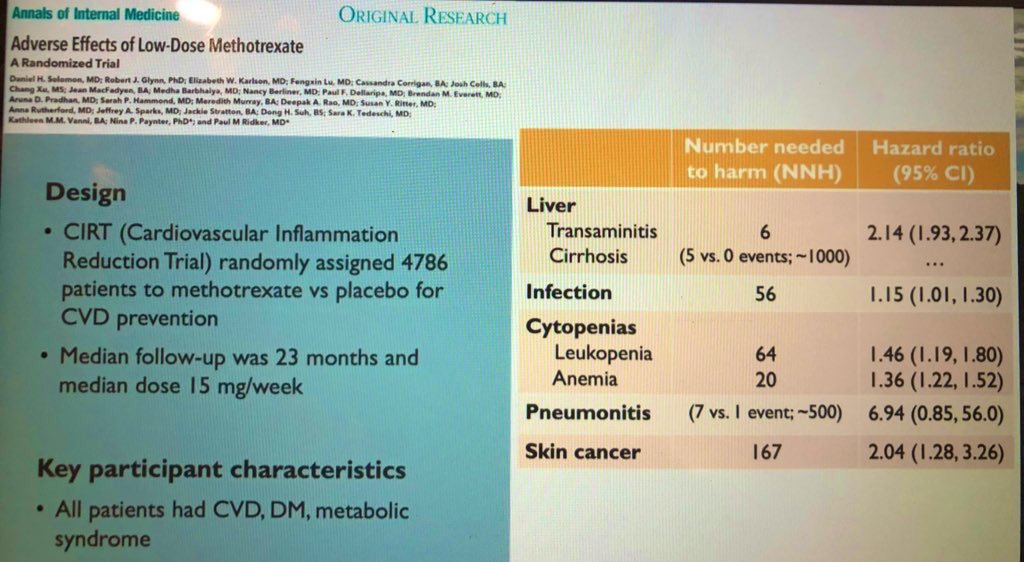
Perform (annual) skin exams in patients in MTX based on discovery of a new adverse effect signal with use of low dose MTX (median dose 15 mg/wk) with a NNH of 167! Screening 160 RA patients on MTX will identify 1 skin Ca. Amazing #ACR20 Year in Review by @JYazdanyMD. @RheumNow https://t.co/hUCzKSYZnB
Meral K. El Ramahi, MD MeralElRamahiMD ( View Tweet)
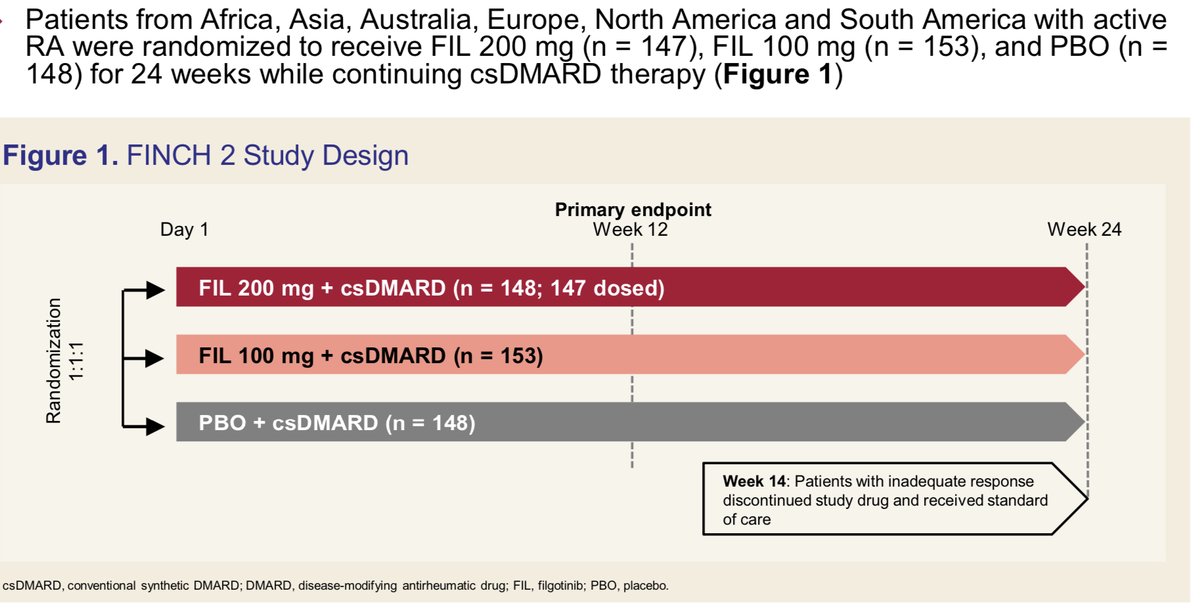
Abst#0142 at #ACR20, a post-hoc analysis of FINCH2, shows that filgotinib (FIL)(Jak1-i) + csDMARD improved the degree of activity impairment in a clinically relevant manner compared to placebo + csDMARD in pts w/ inadequate response to bDMARD . @RheumNow https://t.co/6M42LUVuht
Meral K. El Ramahi, MD MeralElRamahiMD ( View Tweet)
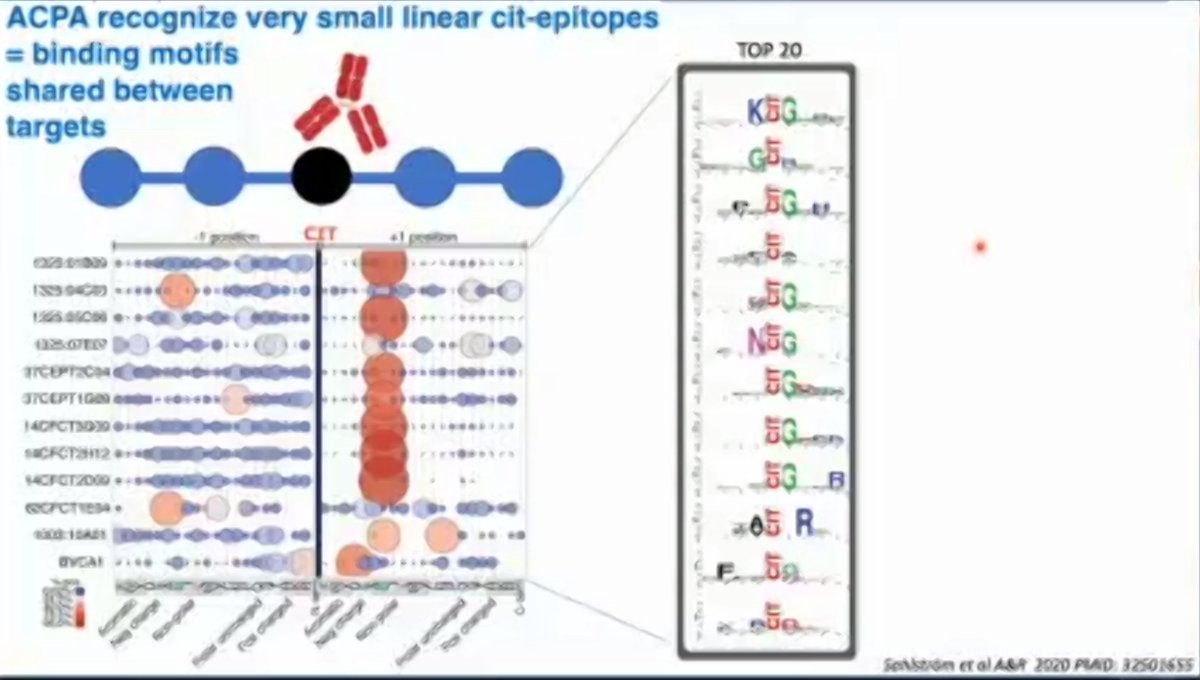
ACPA in patients with RA are multireactive. How can one antibody bind to so many proteins? ACPA recognize very small linear epitopes shared by many posttranslationally modified proteins.
2F012. Antibodies in RA. #ACR20. <TK https://t.co/OUqshFoxSb
ARD & RMD Open ARD_BMJ ( View Tweet)
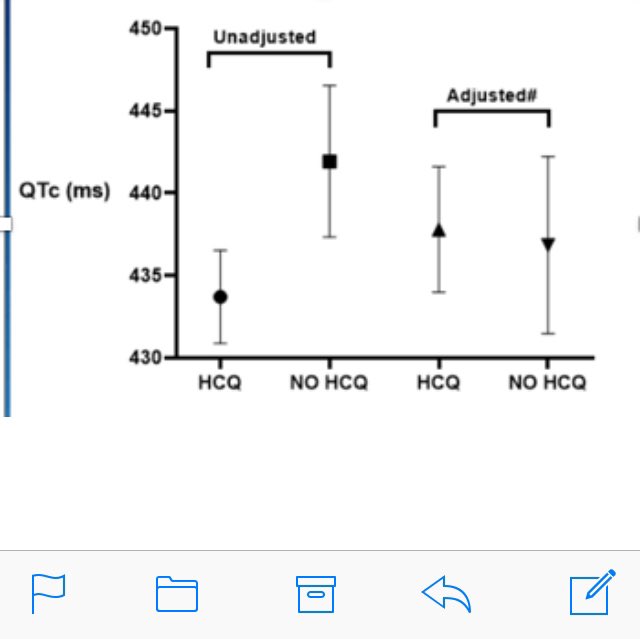
#hydroxychloroquine is not a ‘cutie’ drug. E Park et al showed that HCQ does not increase QT interval in RA & SLE patients including if on other meds that may affect QT but known heart disease excluded from study #ACR20 #ACR2020 @RheumNow abstr#432 @CRASCRRheum https://t.co/FGVPknmj5q
Janet Pope Janetbirdope ( View Tweet)
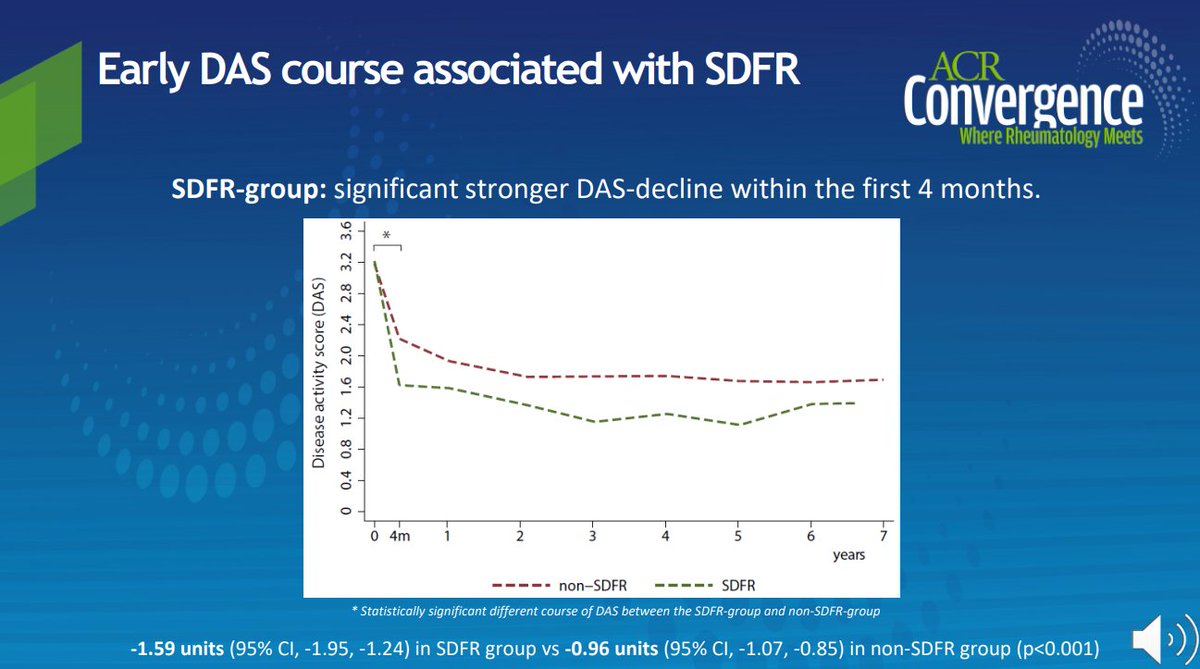
Sustained DMARD-free remission (SFDR) seen in ACPA- RA pts; DAS levels at 4mos. predictive for SFDR. ACPA+ pts no relation w/ SFDR & DAS course @RheumNow #ACR20 Abstr#479 #ACRbest https://t.co/kko4tO7s1Y
sheila RHEUMarampa ( View Tweet)

Large data from Japanese Ninja database in abst#0183 shows incidence of malignancies in Japanese RA patients similar to Japanese gen pop. Comparable in patients exposed and unexposed to biologics/JAKs. #ACR20 @RheumNow
Eric Dein ejdein1 ( View Tweet)

Great debate by Dr. Weinblatt and Dr. Strand on JAKinibs vs TNFi in RA! How long do you tx with JAKinibs before calling it a failure? @RheumNow #ACR20 #ACRbest
Robert B Chao, MD doctorRBC ( View Tweet)
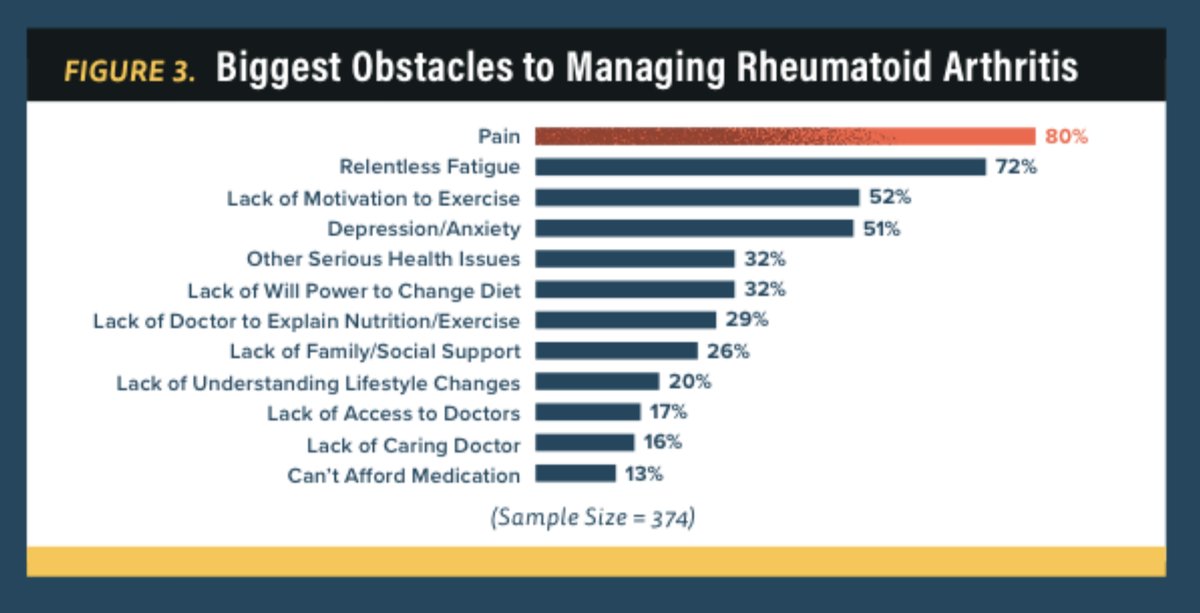
Abst#0135 at #ACR20 illustrates the drivers of patient satisfaction per a survey of 374 U.S. pts. The biggest obstacles to managing RA are pain (80%), fatigue (72%), & depression/anxiety (51%), but only 51% of pts feel that these are addressed. @RheumNow https://t.co/I2ac2T2TOA
Meral K. El Ramahi, MD MeralElRamahiMD ( View Tweet)

#GREATDEBATE Dr. Weinblatt won - but based on the limited # people polled d/t difficulties with website #ACR20 @RheumNow
k dao KDAO2011 ( View Tweet)
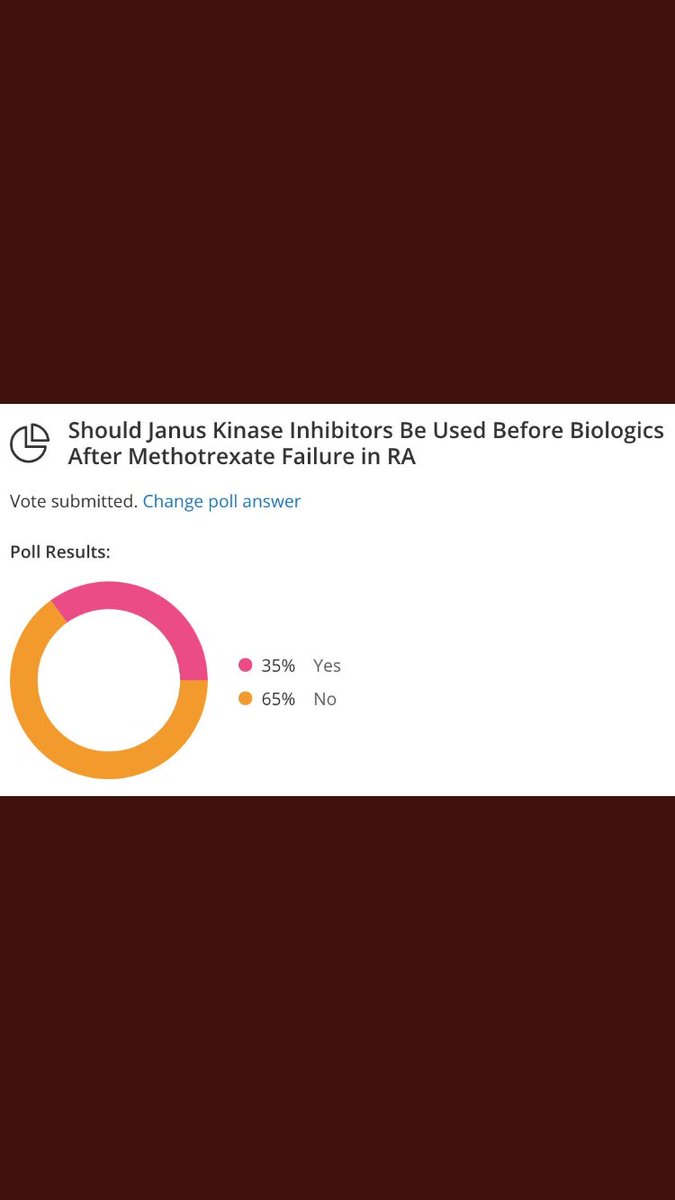
The Great Debate results! https://t.co/tJk0yIRqfK
Dr. Rachel Tate uptoTate ( View Tweet)

Since you’ve just listened to The Great Debate, what do you use as your first line therapy in RA #ACR20? @RheumNow
Dr. Rachel Tate uptoTate ( View Tweet)

RheumNow’s expanded coverage of the #ACR20 Annual meeting is sponsored in part by BMS, @HorizonNews , @SanofiGenzyme, @Novartis. All content chosen by RheumNow & its Faculty.
Dr. John Cush RheumNow ( View Tweet)
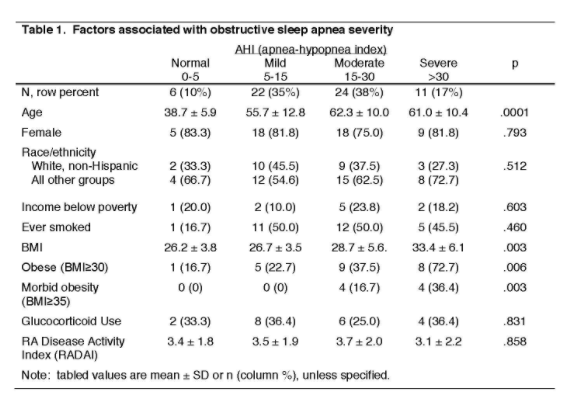
How well do your RA patients sleep? Is OSA an issue?
Validated questionnaires and clinician questioning missed two-thirds (23/35) of mod-severe OSA, no clues from steroid use or RA disease activity.
Should we be screening more patients?
@UCSFHospitals #ACR20 ABST0196 @RheumNow https://t.co/kibwDPYYn9
David Liew drdavidliew ( View Tweet)
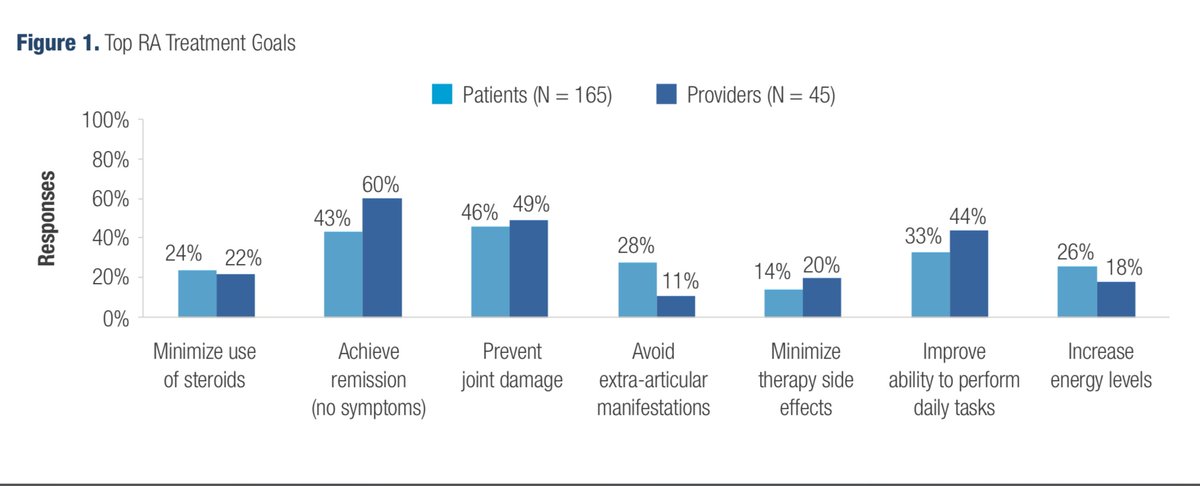
Abst#0138 at #ACR20 shows the importance of identifying alignments & discordances in perceptions of RA tx between providers & patients, individualizing txs accordingly, & a need to engage in shared-decision making to optimize RA care. @RheumNow https://t.co/dTj0S7Zm5x
Meral K. El Ramahi, MD MeralElRamahiMD ( View Tweet)
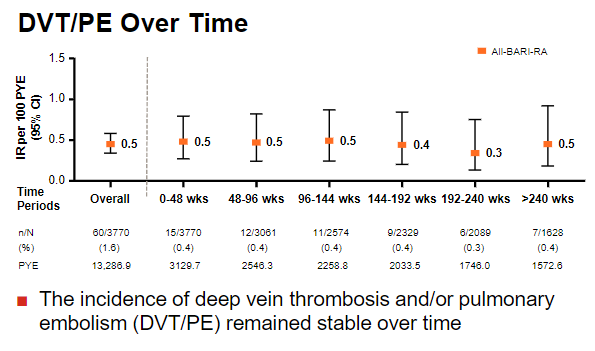
DVT/PE has been the sticking point for baricitinib in RA.
DVT/PE risk in integrated safety analysis >8y: incidence doesn't increase, but also doesn't decrease. No diff with dose either.
Every drug has pros/cons; it's how we manage risk that counts...
#ACR20 ABST0202 @RheumNow https://t.co/V88tQjcXSJ
David Liew drdavidliew ( View Tweet)



